- Home
- -
- ASAM Criteria
- -
- Software
- -
- ASAM CONTINUUM™
- -
- User Release Notes User Release Notes
Release Notes – April 2025
Announcing: The ASAM CO-Triage® Third to Fourth Edition Crosswalk
Enhancing ASAM Criteria Third Edition Software for the Future
For the first time, substance use disorder treatment programs and referrers can gain concrete, objective insight into what changes might be needed to prepare for The ASAM Criteria, Fourth Edition
ASAM and its technology partner, FEI Systems, are completing a new report enhancement to the Third Edition ASAM CO-Triage
The Third to Fourth Edition Crosswalk, now part of the CO-Triage Report, outputs the provisional ASAM Criteria Fourth Edition Level of Care that is closest to the calculated Third Edition Level
The CO-Triage Report Crosswalk will show what provisional Fourth Edition Level of Care the patient might need for comprehensive assessment per The ASAM Criteria Fourth Edition
Providers will be able to learn, for each given patient, how provisional level of care recommendations may be different in the Fourth Edition
Program leaders, administrators, contract/licensing officials, and payers will learn, for the treatment system, how to prioritize the transition to Fourth Edition Levels of Care
The CO-Triage Report Crosswalk does not take the place of the Fourth Edition CO-Triage, but rather provides a bridge until the Fourth Edition CO-Triage is available
The ASAM CO-Triage is the ASAM-endorsed, quick structured interview and decision engine for determining a provisional Level of Care recommendation of where to refer patients with substance use disorders to undergo a comprehensive evaluation.
Example output:

User Release - Previous Updates
Narrative Report Updates
1. A customer reported concerns about output for question ASm03D in the Narrative Report – Problem list, citing issues with their PHI protocols.
Question ASm03d, which appears in the Medical History section of the CONTINUUM interview, asks if the patient has HIV or AIDS, and if so, if they need medical interventions that require a residential setting:
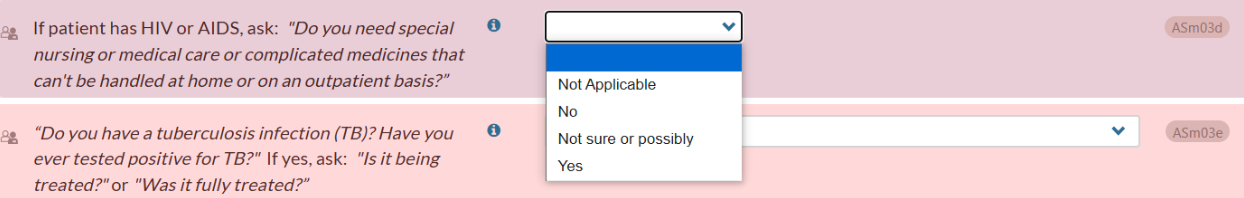
This question and response both output in the Narrative Report – Problem List, Dimension 2: Biomedical Conditions and Complications.
If the response is “No,” “Not sure or possibly,” or “Yes,” the Narrative Report output indirectly confirms the patient’s HIV positive status, which may conflict with some agencies’ PHI protocols.
To address the issue, we removed the first part of the question – “If patient has HIV or AIDS, ask” – from the Narrative Report Problem List output. Now, the output will include only the part of ASm03d that asks if the patient needs special nursing, medical care, or complicated medicines that cannot be handled at home or on an outpatient basis: This update retains useful information for treatment planning while maintaining patient confidentiality and compliance with privacy protocols.

Summary Report Updates
1. In response to a Help Desk ticket, we have updated the Dimensional Analysis section of the Summary Report to more concisely describe the purpose of the Dimensional Analysis grid. We have eliminated the previous text which detailed the decision rules met within each dimension, since that caused some confusion regarding the final Level of Care recommendation.
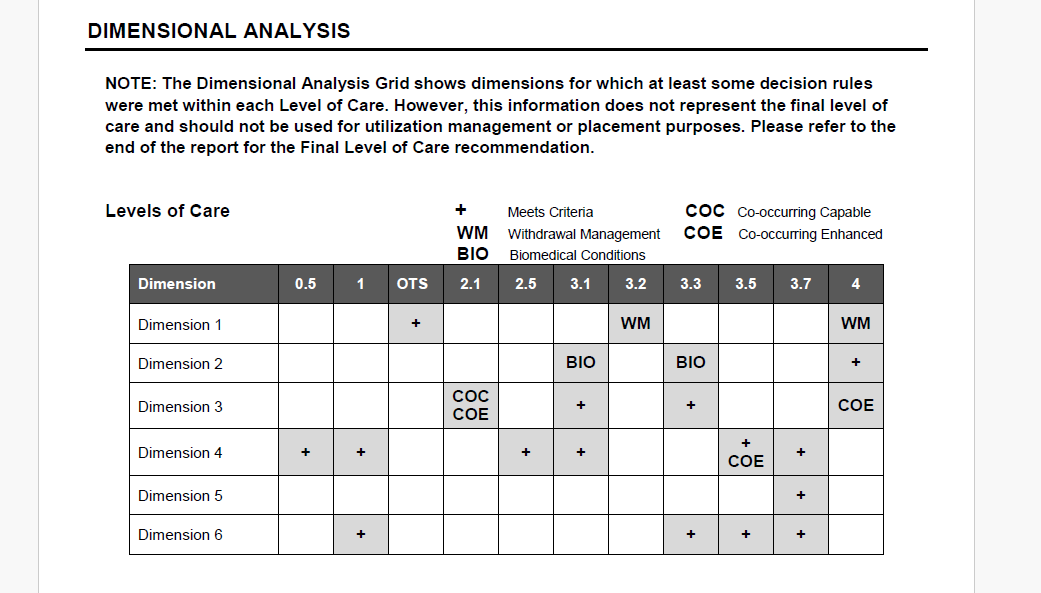
- In the Summary Report Critical Items section, we have revised the output for ASm03e. Previously, when a patient endorsed having a current tuberculosis infection, the output stated (regardless of treatment status) that the patient may need a medical evaluation due to possible tuberculosis infection. Now, the output more clearly specifies treatment needs through improved alignment with the two response options for ASm03e that indicate current tuberculosis infection.
- For question ASm03e: “Do you have a tuberculosis infection (TB)? Have you ever tested positive for TB?” (If yes, ask: "Is it being treated?" or "Was it fully treated?")
If the answer is:
“Yes, known infection which is being or has been medically treated,”
the output will be:
“The patient has or had a tuberculosis infection which is being or has been medically treated.

If the answer is:
“Yes, known or suspected infection that is not being or has not been treated,”
the output will be:
“The patient has a known or suspected infection that is not being or has not been treated; therefore, consider whether the patient might benefit from a medical evaluation.”

Release Notes – June 2024
CONTINUUM Interface Updates – Medical History Section
- In response to user feedback, we have updated the Medical History section HIV and TB test questions (ASm03cRn and ASm03eRn). These questions now include a parenthetical statement at the end of each question: "Leave blank if unsure." Importantly, leaving ASm03cRn and ASm03eRn blank will not impact the Final Level of Care recommendation.


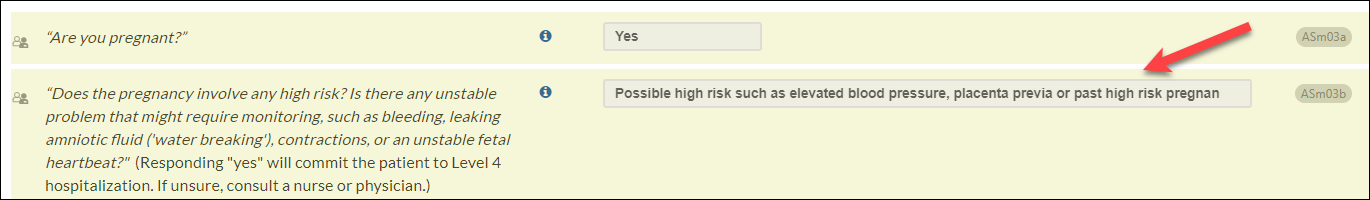
- In response to user feedback, we also have updated the Medical History section questions about assessing blood pressure and heart rate (CIWABPa & CIWAhrs). We have added information icon text for CIWABPa and CIWAhra, stating, "If unable to assess, select 'No.'" This response may apply when users are unable to measure vital signs (e.g., due to telehealth).
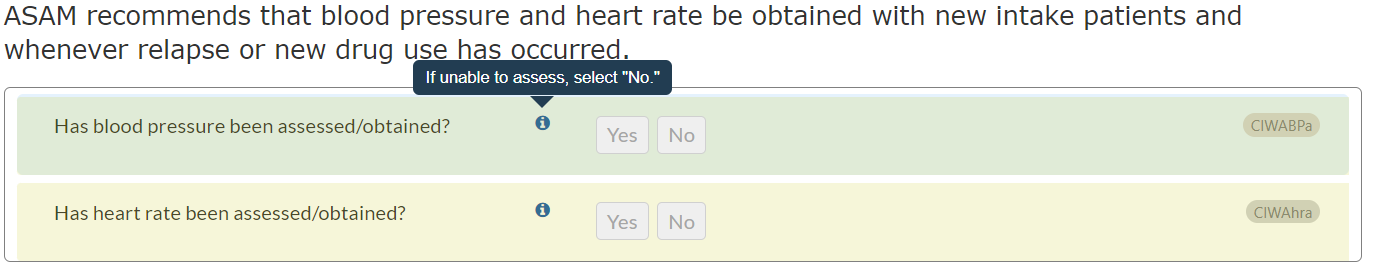
- We have additionally updated the answer choices for question ASm03b for increased clarity.
- If current pregnancy is endorsed in question ASm03a (“Are you pregnant?”), ASm03b will appear:
- Does the pregnancy involve any high risk? Is there any unstable problem that might require monitoring, such as bleeding, leaking amniotic fluid ('water breaking'), contractions, or an unstable fetal heartbeat?
- Question ASm03b includes guidance that responding 'yes' will result in Level 4 hospitalization:
- (Responding “yes” will commit the patient to Level 4 hospitalization. If unsure, consult a nurse or physician.)
- However, two of the answer choices for ASm03b began with 'yes,' introducing potential confusion. We have updated the second answer choice to clarify that “possible high risk” does not independently produce a Level 4 recommendation:
Previous answer choices for ASm03b:
- No
- Yes, possible high risk such as elevated blood pressure, placenta previa, or past high-risk pregnancy
- Yes, immediately unstable
Updated answer choices for ASm03b (change in bold):
- No
- Possible high risk such as elevated blood pressure, placenta previa, or past high-risk pregnancy
- Yes, immediately unstable
CONTINUUM Interface Update – Drug and Alcohol Section
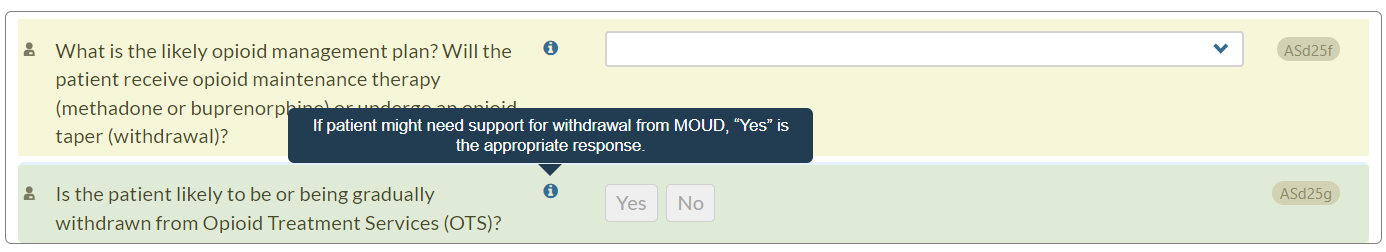
- In the Drug and Alcohol section, Opioid Treatment Services (OTS) subsection, we have added text to the information icon for question ASd25g: "Is the patient likely to be or being gradually withdrawn from Opioid Treatment Services (OTS)?"
Now, when users hover over the information icon, they will see the following guidance: "If patient might need support for withdrawal from MOUD, “Yes” is the appropriate response."
CONTINUUM Interface Updates – Psychological section, Psychological Interviewer Rating subsection
- We have revised the wording of question ASp18b and added text to its information icon. This update, made in response to a Help Desk ticket, aims to clarify the intent of the question since the interviewer may not have been able to interact with the patient during the previous 24 hours.
- Previous question text: Showing fluctuating orientation in the past 24 hours?
- Updated question text (changes in bold): Does the patient show fluctuating orientation at the time of the interview or during the past 24 hours?
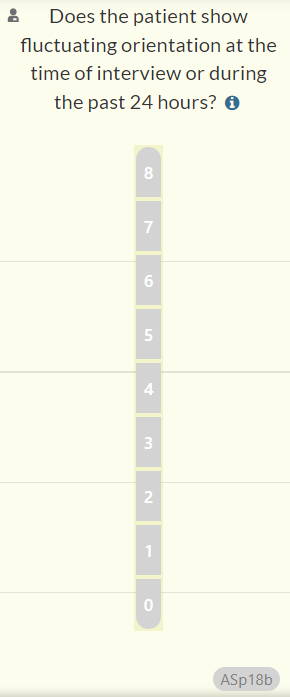
- Information icon: “If no information is available about the past 24 hours, answer based on whether the patient shows fluctuating orientation during the interview.”

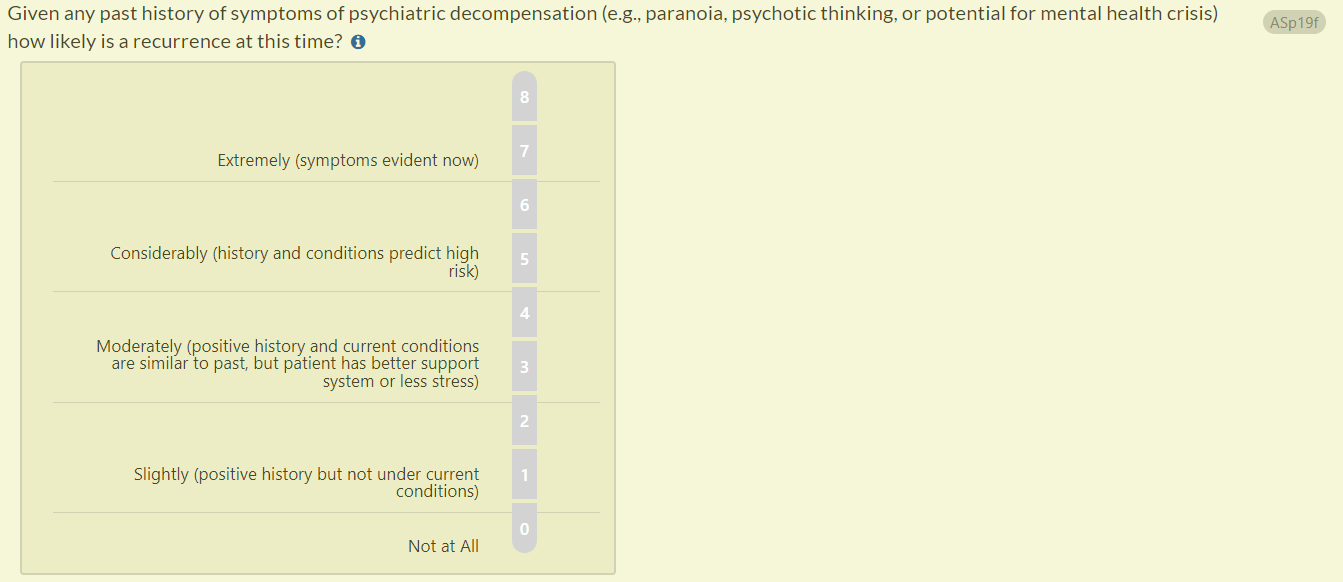
- In response to user inquiries, we have also updated question ASp19f to enhance clarity.
- Previous question text: “Given any past history of symptoms of psychiatric decompensation (e.g., paranoia, psychotic thinking) how likely is a recurrence at this time?”
- Updated question text (changes in bold): “Given any past history of symptoms of psychiatric decompensation (e.g., paranoia, psychotic thinking, or potential for mental health crisis), how likely is a recurrence at this time?”
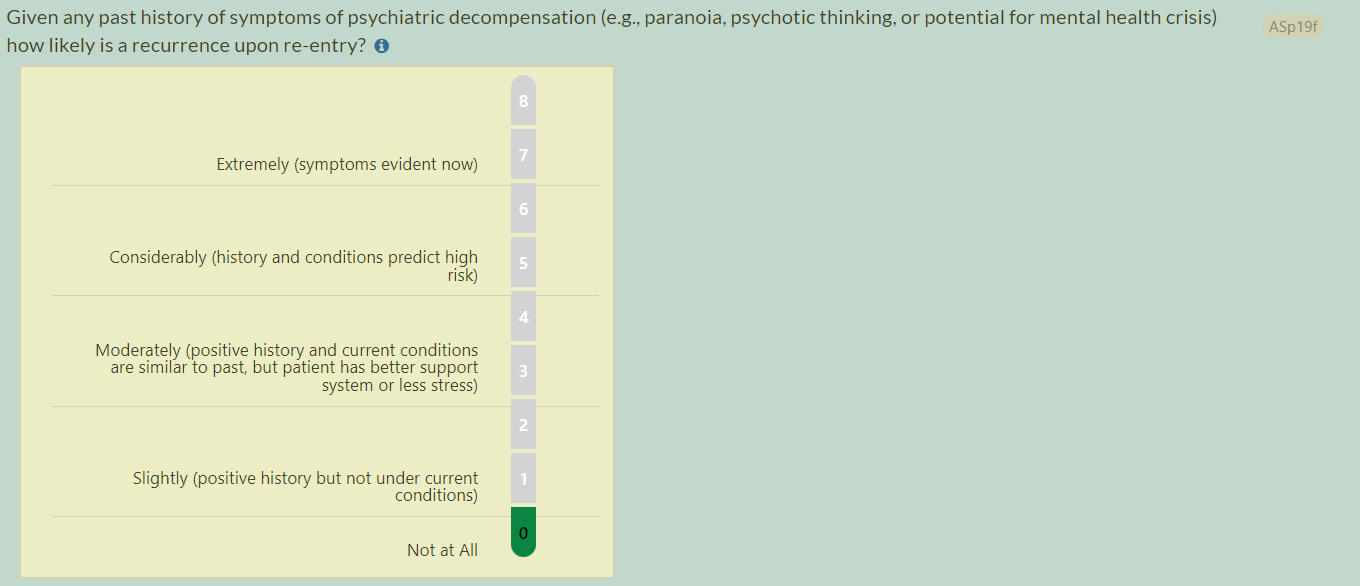
- Previous question text (RISE): Given any past history of symptoms of psychiatric decompensation (e.g., paranoia, psychotic thinking) how likely is a recurrence upon re-entry?
- Updated question text (RISE): Given any past history of symptoms of psychiatric decompensation (e.g., paranoia, psychotic thinking, or potential for mental health crisis) how likely is a recurrence upon re-entry?
- In response to a Help Desk ticket, we have also updated question ASp19k to help interviewers assess cognitive symptoms.
- Previous question text: Does the patient carry or show evidence of a chronic organic mental disability, such as Alcohol Amnestic Disorder (Korsakoff’s Dementia) of Alzheimer’s Disease?
- Updated question text (changes in bold): Does the patient display symptoms indicative of long-term dementia, confusion, or memory loss, or do they have a diagnosis of chronic organic mental disability, such as Alcohol Amnestic Disorder (Korsakoff's Dementia) or Alzheimer's Disease?
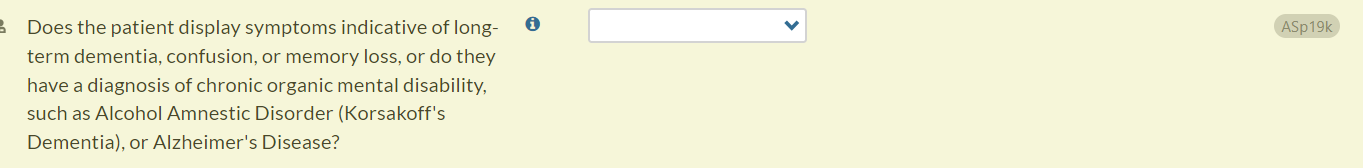
Summary Report Updates
- In the Summary Report, Access to Treatment Issues section, we have updated output for question ASp19k (“Does the patient display symptoms indicative of long-term dementia, confusion, or memory loss, or do they have a diagnosis of chronic organic mental disability, such as Alcohol Amnestic Disorder (Korsakoff's Dementia) or Alzheimer's Disease?”).
- Previously, when the interviewer selected "Not sure or possibly" or "Yes” for question ASp19k, the output stated: "The clinician deduced from the interview or has information that indicated that patient may have a major neurocognitive disorder, a category which includes alcohol amnestic-confabulatory type, or Alzheimer's Disease.”
- The output has been updated so that when the user selects “Not sure or possibly,” it will read: “The clinician is unsure about or has information that the patient may possibly have long-term dementia, confusion, memory loss, or a major neurocognitive disorder, a category which includes alcohol amnestic-confabulatory type, or Alzheimer's Disease.”

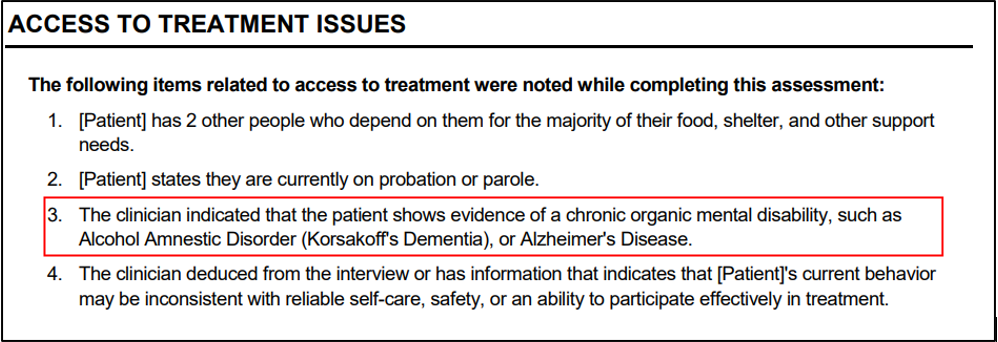
- The output also has been updated so that when the user selects "Yes," it will read: “The clinician indicated that the patient shows evidence of a chronic organic mental disability, such as Alcohol Amnestic Disorder (Korsakoff’s Dementia), or Alzheimer’s Disease.”
- In the Summary Report Critical Items section, we have updated output for ASm03b to support treatment planning.
- If current pregnancy is endorsed in question ASm03a (“Are you pregnant?”), ASm03b will appear: “Does the pregnancy involve any high risk? Is there any unstable problem that might require monitoring, such as bleeding, leaking amniotic fluid ('water breaking'), contractions, or an unstable fetal heartbeat?”
- If the user selects “Possible high risk such as elevated blood pressure, placenta previa” for ASm03b, the following output will appear: “[Patient] indicated a possible high-risk pregnancy.”

- If the user selects “Yes, immediately unstable” for ASm03b, the following output will appear: “[Patient] indicated an immediate unstable pregnancy.

Narrative Report Update
- For patients who receive Medicaid, there is a new option to display date of birth (DOB) and Medicaid ID number in Narrative and Summary Report headers.
In the General Information section, PtIns question, the interviewer can select Medicaid and then enter the patient’s Medicaid ID number in the MdcdIDNo field if known:
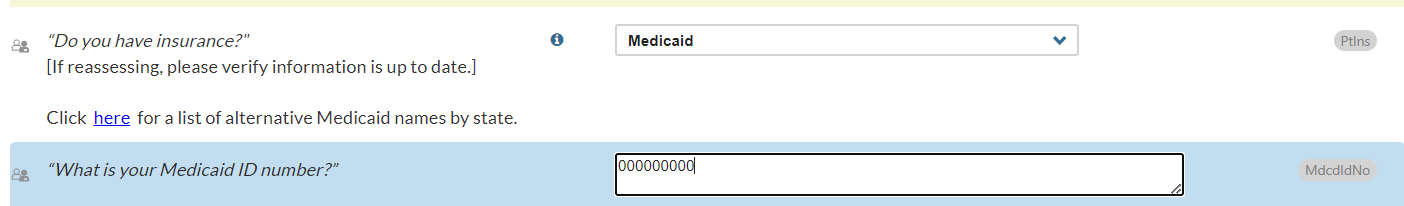
If Medicaid is selected in the PtIns question, the Narrative and Summary Report headers will display the patient’s date of birth and Medicaid ID number (if supplied in the MdcdIDNo field):

If the Medicaid ID number is not supplied, this line will read, “Not provided.”

Release Notes – March 2024
CONTINUUM Level of Care Output – Sustained Remission
The Summary and Narrative Report Final Level of Care Recommendations will now include personalized output for patients in sustained remission from substance use disorder (i.e., they have not met any DSM-5-TR criteria for substance use disorder (besides craving) in the past 12 months.
- NOTE: As per The ASAM Criteria, Level 1 is recommended for ongoing remission monitoring.
- If the patient also needs service coordination for psychiatric, biomedical, or housing needs, the output will specify those needs.

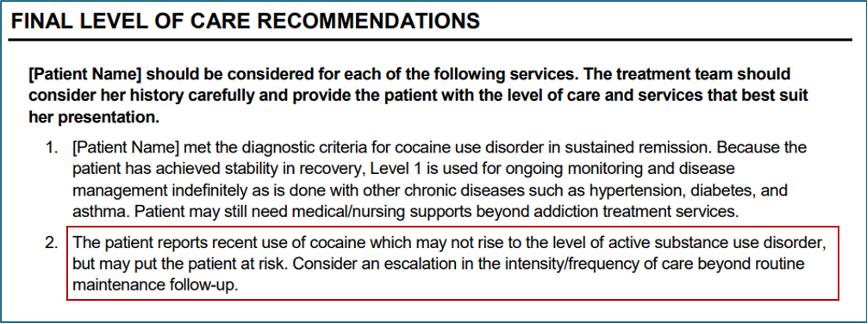
- For patients who meet diagnostic criteria for sustained remission, but report substance use within the past 30 days, the Summary Report’s Diagnostic Findings section will display an asterisk with an associated message: “Recent substance use was reported.” This modification will alert the clinician that recent use may destabilize the patient’s remission.
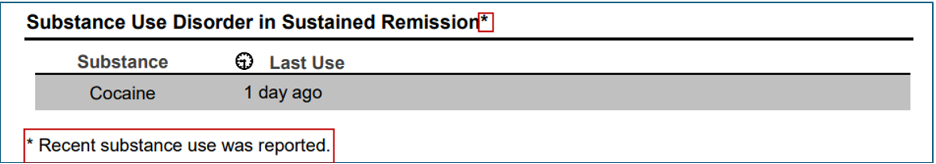
- For patients in sustained remission who have used any substance in the past 30 days, the Final Level of Care Recommendations will also indicate a potential need for escalation beyond Level 1 services:
CONTINUUM Level of Care Output – Level 2.1 + 3.1
We are introducing a new recommendation in the Summary and Narrative Reports, Final Level of Care Recommendations section, for Level 2.1 + 3.1 to further personalize CONTINUUM results.
- This new recommendation is appropriate for patients who meet dimensional admission criteria for Level 2.1 (i.e., they need 9 to 19 clinical service hours per week) but also require a residential component because their living situations or recovery environments are incompatible with their recovery goals.
- Patients may now receive a Final LOC recommendation for:
- Level 2.1+ Level 3.1;
- Level 2.1 Co-Occurring Capable (COC) + Level 3.1; or
- Level 2.1 Co-Occurring Enhanced (COE) + Level 3.1.

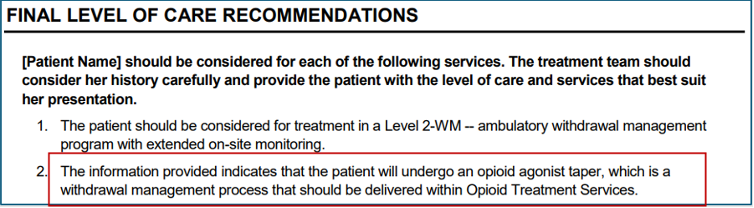
CONTINUUM Level of Care Output – Opioid Treatment Services for MOUD Taper
We are also introducing new output in Final Level of Care Recommendations for Opioid Treatment Services (OTS) for patients who will undergo an opioid agonist taper and need assessment and treatment of associated withdrawal symptoms.
- This new output will provide context for an OTS recommendation when the clinician indicates that a patient will be gradually withdrawn from a medication for opioid use disorder (MOUD) such as buprenorphine or methadone.
Co-Triage Level of Care Output: Level 2 for Withdrawal Management Needs
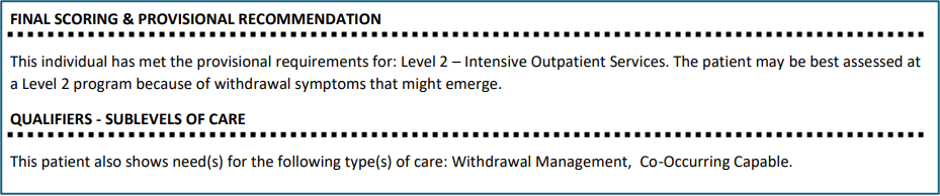
- Patients who would otherwise meet provisional criteria for Level 1 but have withdrawal management needs that require extended nurse monitoring, will now receive a recommendation for Level 2.
- The new output will specify the patient’s need for withdrawal management evaluation at a Level 2 program:
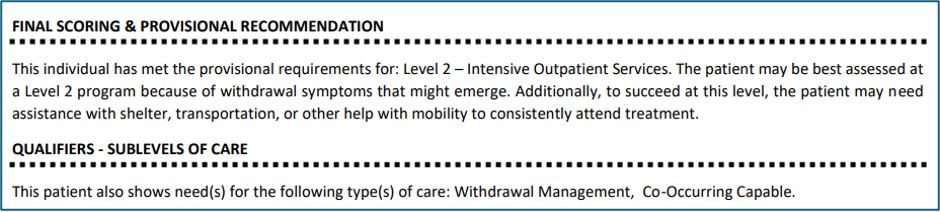
- If applicable, the output will also specify the patient’s need for assistance with shelter, transportation, or mobility to enable outpatient treatment attendance:
- The new output will specify the patient’s need for withdrawal management evaluation at a Level 2 program:
CONTINUUM Narrative/Summary Report Output: Diagnostic Findings
To improve alignment with the DSM-5-TR, we have updated the Narrative and Summary Report Diagnostic Findings table to reflect drug class diagnoses.
- For instance, if a patient meets one criterion in “heroin/fentanyl” and another criterion in “other opioids”, they will receive an opioid use disorder diagnosis.
- The DSM-5-TR Substance Use Disorder(s) table will display this diagnosis under the new “Drug Class” section.
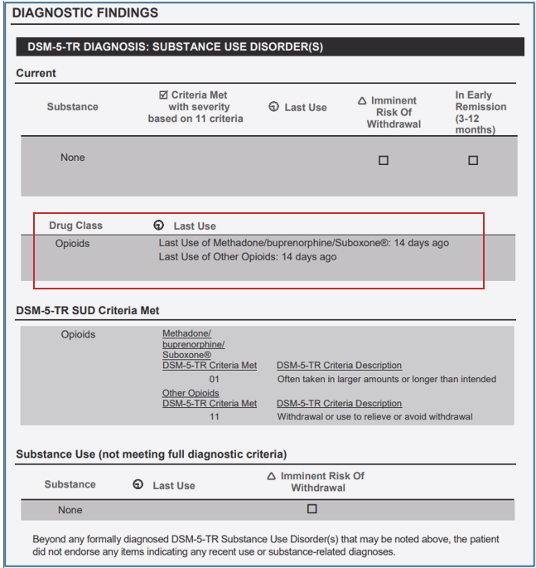
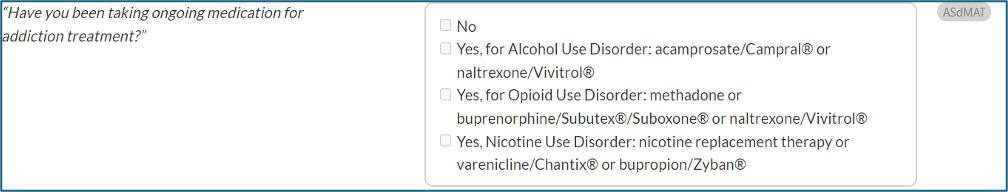
CONTINUUM Interface Update - ASdMAT
- A new question in the Drug and Alcohol section, Addiction Treatment History subsection, prompts interviewers to ask, “Have you been taking ongoing medication for addiction treatment?” (ASdMAT).
- Interviewers will be able to indicate if the patient is taking ongoing medication for alcohol use disorder, opioid use disorder, or nicotine use disorder.
- This question will be used to inform new decision logic for sustained remission diagnoses and associated level of care output.
CONTINUUM Interface Update – Illicit versus prescribed fentanyl (SubsUse)
- In the Drug and Alcohol section, we have refined response options for the SubsUse question: "Which substances have you had problems with?" to better distinguish between illicit versus prescribed fentanyl.
- “Heroin/fentanyl” has been changed to "Heroin/illicit (street) fentanyl."
- The response option for other opioids has been updated to: "Opioid or narcotic other than heroin, methadone, or buprenorphine, even if by prescription, including prescribed fentanyl."

CONTINUUM Interface Update – ASd25f
We have also introduced a new Warning message in the Drug and Alcohol section, Opioid Treatment Services subsection, to emphasize the importance of MOUD (medication for opioid use disorder) as the preferred approach for treating heroin or opioid withdrawal symptoms.
- Users will now receive a Warning message for item ASd25f if they select “Withdrawal management with no medication” AND the patient reports recent, frequent opioid use and/or current opioid withdrawal symptoms.

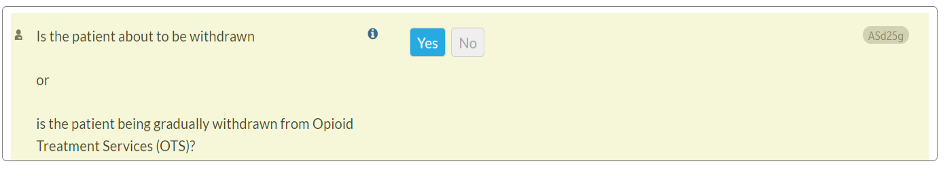
CONTINUUM Interface Update – ASd25g
In response to a help desk ticket, we have revised the wording of question ASd25g within the Drug and Alcohol section, Opioid Treatment Services subsection. This update aims to enhance the accuracy of user responses.
- Previous question text: Is the patient to be or being gradually withdrawn from Opioid Treatment Services (OTS)?
- Updated question text: Is the patient about to be withdrawn or is the patient being gradually withdrawn from Opioid Treatment Services (OTS)? 123
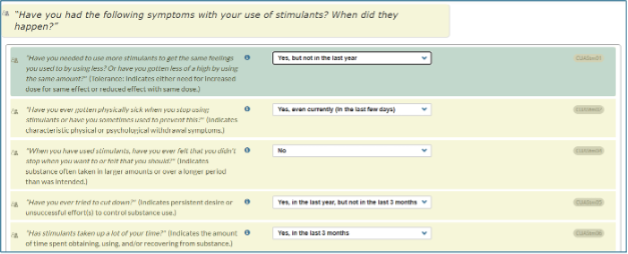
CONTINUUM Interface Update – Revision of DSM-5-TR ItemsWe have revised the DSM-5-TR questions to enable SUD diagnoses of early and sustained remission.
- "Early remission" means that symptoms other than craving have been absent for at least the past 3 months, but less than 12 months.
- "Sustained remission" means that symptoms other than craving have been absent for 12 months or longer.
For each DSM-5-TR criterion, users can select from four options to specify the time frame in which the patient experienced that symptom:
- No
- Yes, but not in the last year
- Yes, in the last year, but not in the last 3 months
- Yes, in the last 3 months
- Yes, even currently (in the last few days) *
* This response, “yes, even currently (in the last few days),” only appears as an option for the DSM-5-TR question related to withdrawal, which asks if the patient has gotten physically sick when they stop using or continued to use to prevent getting sick (CUAXxx02).
Release Notes – Version 3.27 (February 13, 2024)
Update to CONTINUUM Legal Information Section
In response to a Help Desk inquiry, we have streamlined the process of reporting charges and arrests by prepopulating ASl03-ASl15 criminal offense questions with a value of “0”. This enhancement is designed to optimize user efficiency. When “Yes” is chosen for ASl01z (“Have you ever been arrested or charged for any reason?”), the Warning message will prompt the user to update the criminal offense fields with the message, “WARNING: The response indicates the patient has been arrested or charged. Please report specific charges in ASl03 – ASl18 below.”
Release Notes – Version 3.25 (October 24, 2023)
Update to CONTINUUM General Information Section
To provide clinicians with additional data to support care navigation and treatment planning, we have developed a new, optional question to determine the type of insurance a patient has.
- The new insurance options include No, Medicaid, Private commercial insurance (employer-sponsored), Private commercial insurance (non-employer sponsored), Medicare, Tricare, and Other:
- If the patient has Medicaid insurance, a prompt will ask for the Medicaid ID number:
- There is also a new link to an external resource with alternative Medicaid names by state.
- If “Other” is selected, another prompt will ask, “Please specify other insurance”:
- When something other than Medicaid or Other is selected, there will be no additional prompt.
- When an assessment is pulled forward to reassess a patient, it will retain the insurance information and ID number when applicable. Please be sure to double-check with the patient that this information is still accurate.
Update to CONTINUUM Drug and Alcohol Section, Opioid Treatment Services Subsection
Per CONTINUUM user feedback, question ASd25f (Will the patient be treated using an opioid withdrawal protocol?) will be reworded to help interviewers differentiate between opioid agonist tapers versus ongoing use of opioid agonists to support OUD recovery.
- The updated question will appear as follows: What is the likely opioid management plan? Will the patient receive opioid maintenance therapy (methadone or buprenorphine) or undergo an opioid taper (withdrawal)?
- The first response option for ASd25f has been updated from "Not applicable - the patient will start or remain on opioid maintenance treatment (e.g., methadone, buprenorphine)" to "No taper/withdrawal - the patient will start or remain on opioid maintenance treatment (e.g., methadone, buprenorphine)."
- This revision clarifies that the patient will not undergo a taper or withdrawal process, but rather initiate or continue MOUD (medications for opioid use disorder).
- This revision clarifies that the patient will not undergo a taper or withdrawal process, but rather initiate or continue MOUD (medications for opioid use disorder).
Release Notes – Version 3.24 (September 12, 2023)
Update to CONTINUUM Psychological Section, Psychological Interviewer Rating Subsection
- CONTINUUM is discontinuing the use of the Global Assessment of Functioning (GAF) scale, as it is no longer supported by the American Psychiatric Association (APA). We are shifting to the Symptom-Function Scale (SFS), a new 5-point scale. This scale ranges from 0 (in dark green, below) to 4 (in red). The scale appears in the Psychological Interviewer Rating section, in new required question SxFctSc.
- When determining the SFS score, clinicians should consider the patient's psychological, social, and vocational/educational symptoms and functional issues, if any. Then, they should rate the severity of the patient's most concerning issue(s) among these, excluding functional impairment due to physical or environmental limitations (e.g., inability to ambulate without a cane).
- When determining the SFS score, clinicians should consider the patient's psychological, social, and vocational/educational symptoms and functional issues, if any. Then, they should rate the severity of the patient's most concerning issue(s) among these, excluding functional impairment due to physical or environmental limitations (e.g., inability to ambulate without a cane).
- Narrative and Summary Report: The Symptom-Function scale output will replace the GAF output in both the Narrative and Summary reports, as shown below.
- The selected response will appear first, followed by the full SFS score range for reference.
Update to CONTINUUM, Psychological Section, Psychological Interviewer Rating Subsection
- The selected response will appear first, followed by the full SFS score range for reference.
- In response to a Help Desk inquiry, we have revised question ASp20c to clarify that it refers to mental health needs. This question aims to determine whether continuous 24-hour psychiatric monitoring is needed to ensure treatment adherence and effectively address challenges such as ambivalence towards psychiatric medications and participation in a recovery program. The level of supervision needed to prevent risky substance use is derived from other questions in the Drug & Alcohol section, among others.
- Previously, the question read: Will the patient require any treatment modalities that require a 24-hour controlled, supervised environment?
- Now, the question reads: Will the patient’s mental health needs require a 24-hour controlled, supervised environment?
Update to CONTINUUM Summary Report
- The "Possible Non-Substance Use Disorder Psychological Conditions" box has now been moved above the Withdrawal Scales section, because it was previously appearing as part of that section. It will now appear below the “Diagnostic Findings” section.
Updates to CONTINUUM Narrative Report, Psychiatric Section
We have made changes to facilitate clinical focus on the most acute psychiatric symptoms displayed in the Narrative Report Psychiatric section, as follows:
- When suicidal and other psychiatric symptoms are endorsed, suicidal symptoms will be presented before other psychiatric symptoms in the Narrative Report Psychiatric section, “Serious Emotional and Psychological Problems - Lifetime” subsection.
- This subsection will also display thoughts of self-harm (ASp08a: "Thoughts of how you might hurt yourself?") when endorsed.
Update to CONTINUUM Narrative Report, Psychiatric Section
- Previously, when an interviewer endorsed that the patient had reported suicidal thoughts, cognitive distortions or trouble comprehending, the CONTINUUM Narrative Report Psychiatric section would output that they were “clearly” experiencing symptoms. However, this output would display even if the interviewer had selected the response option “Slightly symptomatic (patient reports symptoms, no impairment of behavior or function),” in which case there was no observable impairment.
To improve accuracy, and in response to a Help Desk inquiry, the word “clearly” has now been removed from Narrative Report output for question ASp17 (Having trouble with reality testing, thought disorders, paranoid thinking?), ASp18 (Having trouble comprehending, concentrating, remembering?), and ASp19 (Having suicidal thoughts?).
- Previously, when an interviewer endorsed that the patient had reported suicidal thoughts, cognitive distortions or trouble comprehending, the CONTINUUM Narrative Report Psychiatric section would output that they were “clearly” experiencing symptoms. However, this output would display even if the interviewer had selected the response option “Slightly symptomatic (patient reports symptoms, no impairment of behavior or function),” in which case there was no observable impairment.
Release Notes – Version 3.23 (August 1, 2023)
Updates to CONTINUUM Narrative and Summary Report, Diagnostic Findings Section
- The Drug use table found in the Summary Report has now also been added to the Narrative Report.
- It is possible that a patient could be at risk of imminent withdrawal from a substance even if the patient does not actually meet a DSM-5 substance use disorder diagnosis. This can occur with the use of prescribed medications that induce physiologic dependence. In such cases, the Narrative and Summary Reports will now show an alert in the Diagnostic Findings section, within the Drug Use table. Specifically, an “Imminent Risk of Withdrawal” column has been added to the Drug Use table, and a check will appear in the box when a patient is at imminent risk of withdrawal. Previously, imminent risk of withdrawal was only indicated when a patient also met the criteria for a substance use disorder shown by a check in the DSM-5-TR Diagnosis: Substance Use Disorder(s) table.
- Please see the following screenshot displaying an example of a person at imminent risk of withdrawal from alcohol, who does not have an alcohol use disorder diagnosis:

Update to CONTINUUM Interview, Legal Information Section
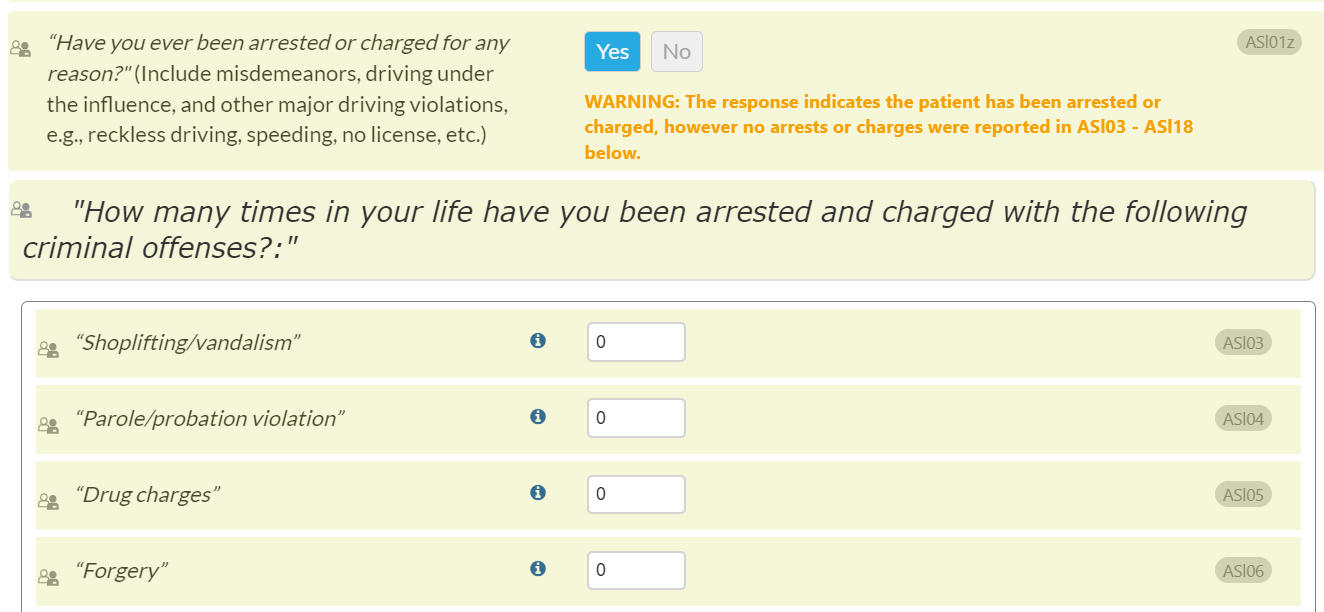
- For data consistency, a new warning message has been added to the Legal Information Section. When “yes” is selected for the question, "Have you ever been arrested or charged for any reason?" (ASl01z), but zero is entered for all of the questions ASl03 - ASl18 (e.g., "Shoplifting/vandalism", "Parole/probation violation", "Drug charges", etc.) OR zero is entered for ASl03 – ASl14c and ASl16 – ASl18 are left blank. This will prompt the interviewer to check and assure that the entered information is consistent.
- Message text will read: WARNING: The response indicates the patient has been arrested or charged, however no arrests or charges were reported in ASl03 - ASl18 below.
- Please see the following screenshot of the new warning message where zero was entered for ASl03 - ASl18:
Update to CONTINUUM Narrative Report, Interviewer Impressions and Recommendations Output
- To be more patient-centric and avoid assumptions that could inadvertently imply intent, we have modified the question pattern regarding patient misrepresentation of information to be more objective. This occurs in the Narrative Report “Interviewer Impressions and Recommendations” output for all the following Sections: Medical, Employment, Alcohol & Drug, Legal, Family/Social, and Psychiatric. Please see the following change below from the red highlighted wording to the green:
- Previously: It is my impression that [Patient] understood all of the questions, and that she did not deliberately misrepresent information about her drug or alcohol use and history.
- Now: I think [Patient] understood all of the questions and did not significantly misrepresent information about her drug or alcohol use and history.

Updates to CONTINUUM Interview, Psychological Section
In the Psychological section, Psychological Interviewer Rating subsection, we have added a new response option to question ASp19h (“Is the patient able to safely access the community for work, education, and other community resources?”).
- Previously the interviewer could select “No or not applicable” or “Yes.”
Now there will be three response options: “No,” “Yes,” or “Not applicable.”
- “Not applicable" implies that the patient may not be able to independently access the community, but is not at risk because:
- The patient has a caregiver to enable safe community access, or
- The patient does not need to access the community (e.g., because the patient is currently in a residential program/controlled environment, or does not currently work, go to school, or use social services).
Standard CONTINUUM:
- The new response option “Not applicable” includes the following statement in parentheses:
- Not applicable (e.g., patient does not need to access the community because they are in a controlled environment or protected transport can be arranged)

CONTINUUM RISE:
- The new response option “Not applicable” includes the following statement in parentheses:
- Not applicable (e.g., patient will not need to access the community because they will be in a controlled environment or protected transport will be arranged)

Additionally, we have updated the question hint/help text for question ASp19h.
- Previously, this statement referred to the patient’s independent ability to access the community. The word “independent” was removed to acknowledge that some patients have caregivers who can help them to safely access the community. The statement will now appear as follows:
Answer based on the patient’s ability to access the community. Use both the patient’s response & all available objective data to draw a conclusion.

CO-Triage Report: New Critical Item for Suicidal Ideation
In response to a Help Desk inquiry, we have added a critical item alert for suicidal ideation to the CO-Triage report.
- For question TASp08D (“Are you having serious thoughts of suicide?”) a new critical item will be displayed in the CO-Triage report when any of the following response options is selected:
- “Symptoms present, with impairment and/or imminent risk BUT related only to intoxication”
- “Symptoms present, with impairment and/or imminent risk BUT related only to substance withdrawal”
- “Symptoms present, with impairment and/or imminent risk but not related to substance use or withdrawal”
The alert will appear as shown in the following screenshot:

Updates to CONTINUUM Narrative and Summary Reports
- Narrative Report Dimension 3 Problem List:
To support treatment planning, we have expanded the selection of Dimension 3 issues reported in the Narrative Report Problem List in the ASAM Dimension 3 - Emotional, Behavioral, or Cognitive Conditions and Complications section.
- To facilitate focus on current Dimension 3 symptoms, only the most recent occurrence of Dimension 3 issues endorsed during the interview will appear in the Narrative Report Problem List. For instance, if the patient endorses ASp03 (Feeling bad about yourself, like a failure, or have let others down?) for both the past month and past 24 hours, only the past 24-hour occurrence (ASp03cD) will output.
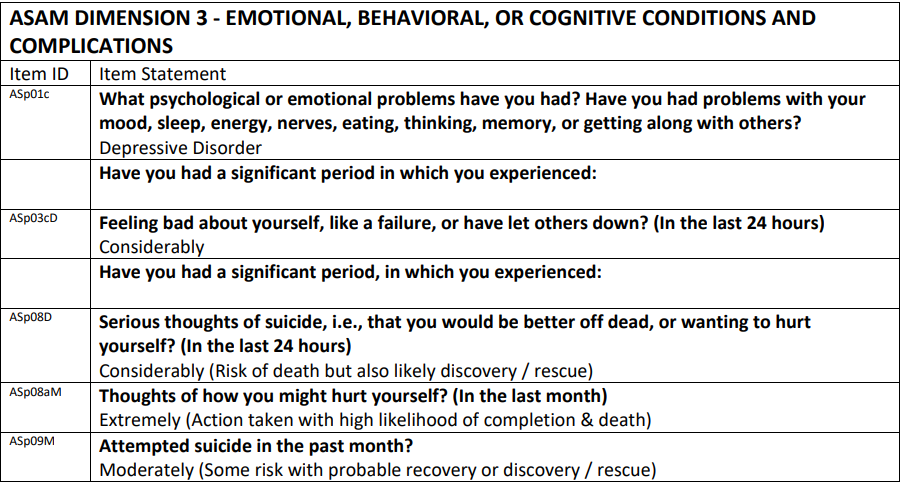
- Most Dimension 3 items will only be included in the Problem List if endorsed as “Considerably” or “Extremely” (rather than “Slightly” or “Moderately”). This is done to help clinicians prioritize immediate treatment needs, since “Slightly” and “Moderately” response options for most Dimension 3 questions specify that there is no current impairment.
- Only ASp19f (Given any past history of symptoms of psychiatric decompensation (e.g., paranoia, psychotic thinking) how likely is a recurrence at this time?) will display in the Dimension 3 Problem List when it is endorsed as “Moderately,” “Considerably” or “Extremely.” This is done to advise clinicians of possible near-future need for skilled mental health interventions, as the response option “Moderately” for this item specifies “positive history and current conditions are similar to past, but patient has better support system or less stress.”
- If no Dimension 3 problems are reported, the following updated message will display: In this Dimension, the patient's assessment did not reveal severe psychiatric symptoms requiring immediate attention. The updated language is intended to clarify that the patient may have a history of less severe and/or less recent psychiatric symptoms that do not appear in the Dimension 3 Problem List, since the Problem List highlights the most recent severe symptoms in need of immediate treatment.

- Narrative Report Psychiatric section, “Severity of Emotional and Psychological Problems and Desire for Treatment”
We have made several changes to facilitate clinical focus on the most acute psychiatric symptoms displayed in the Narrative Report Psychiatric section, as follows:
- When suicidal and other psychiatric symptoms are endorsed, suicidal symptoms will be presented before other psychiatric symptoms in the Narrative Report Psychiatric section, “Severity of Emotional and Psychological Problems and Desire for Treatment” subsection.
- This subsection also will now display past 24-hour and past 30-day suicidal plan and ideation (previously, only suicide attempt displayed in this section).
- When a suicidal symptom is endorsed, only the most recent occurrence (24 hours, past 30 days, or lifetime) will output.
- If more than one suicidal symptom is endorsed within a given timeframe (i.e., past 24 hours), these symptoms will be displayed in order of severity (attempt will appear before plan; plan will appear before ideation).

- Summary Report Critical Items
We have made several changes to facilitate clinical focus on the most acute psychiatric symptoms displayed in the Summary Report Critical Items, as follows:
- When psychiatric symptoms are endorsed in the assessment, only the most recent time frame (24 hours, past 30 days, or lifetime) for each symptom will appear in the Summary Report Critical Items. For example, if hallucinations are endorsed within the past 24 hours and past month, only the past 24-hour time frame will display for that symptom.
- When suicidal symptoms and/or other psychiatric items are endorsed within the past 24 hours, suicidal symptoms will appear before other psychiatric items in the Summary Report Critical Items.
- If more than one suicidal symptom is endorsed within a given timeframe (e.g., attempt, plan, and ideation are all endorsed within the past 24 hours), these symptoms will be displayed in order of severity (attempt will output first, followed by plan, then ideation).
- When a suicidal symptom (attempt, plan, and/or ideation) is reported, it will be capitalized in the Summary Report Critical Items as follows, for all time frames:
[Patient Name] ATTEMPTED SUICIDE, THOUGHTS OF HOW [HE/SHE/THEY] MIGHT HURT [HIMSELF/HERSELF/THEMSELVES], SERIOUS THOUGHTS OF SUICIDE.
Release Notes – Version 3.20 (March 24, 2023)
CO-Triage/CONTINUUM
Alcohol and Drug Interviewer Rating subsection
Now when a CO-Triage assessment is pulled forward into a CONTINUUM assessment, the interviewer’s response to the withdrawal management question in CO-Triage (TWSxs : “Based on your experience, would you need a detox to stop using?” will no longer auto-populate for the corresponding CONTINUUM item ( ASd99: Is the patient experiencing signs and symptoms of withdrawal, or is there evidence that withdrawal is imminent?). This change will require the interviewer to provide the most up-to-date information regarding the patient’s withdrawal symptoms.
The reason for this change is that withdrawal symptoms can frequently change, sometimes as frequently as hourly. Therefore, pulling forward data that may already have changed is not optimal clinical practice.
TWSxs (CO-Triage) 
ASd99 (CONTINUUM):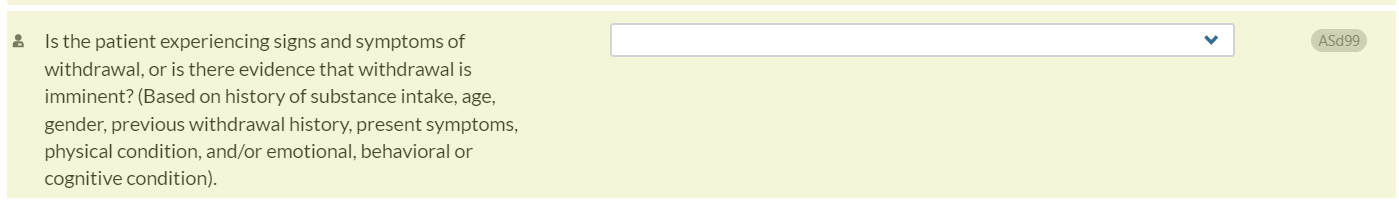
CONTINUUM/CO-Triage Support Form
Interviewer’s Name Now Added to Customer Support Form:
When submitting a support ticket in CONTINUUM/CO-Triage, interviewers will now be able to add their names to the form
. This will help customer support communicate more effectively with CONTINUUM users who submit support tickets. More than one name and email can be added to the support forms.
CONTINUUM/CO-Triage Items
OTP Level of Care Option Added:
Some CONTINUUM interview questions require the interviewer to specify a Level of Care. For example:
- ASd21a: "In the past 90 days, have you relapsed after being discharged from, or dropping out of, another treatment program? What type?"
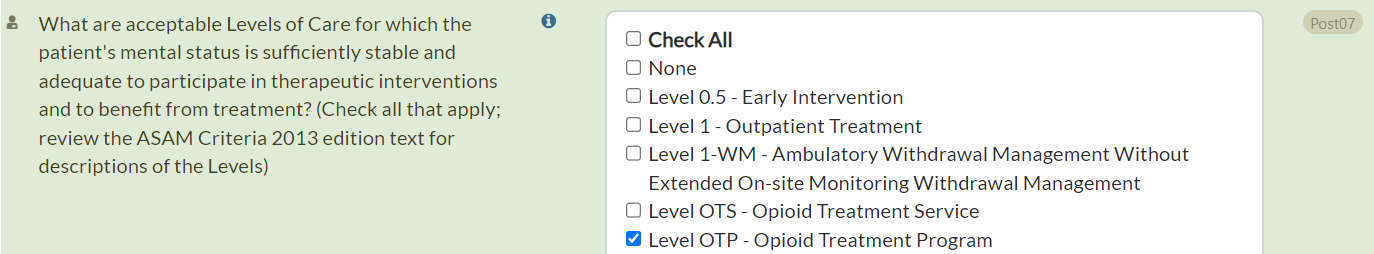
For these items that ask about a Level of Care (including ASd21a, Post07, Post08, Post09, Post11, Rvw14, and Rvw14a), we have now added another option: Opioid Treatment Program (OTP). This now appears after Opioid Treatment Service (OTS).
In CO-Triage, the new OTP option has been added to Rvw14 and Rvw14a.
OTP refers to a federally regulated methadone program, while OTS refers to office-based MOUD (medications for opioid use disorder) treatment.
- ASd21a: "In the past 90 days, have you relapsed after being discharged from, or dropping out of, another treatment program? What type?”
- Post07: What are acceptable Levels of Care for which the patient's mental status is sufficiently stable and adequate to participate in therapeutic interventions and to benefit from treatment?
- Post08: "Are any particular treatment settings unacceptable to you?"
- Post09: "Are there any settings of care that might be unavailable to you because of travel or insurance problems?"
- Post11: Given what you know about this patient, what best treatment(s) would you recommend at this time?
- Rvw14: Category of primary final disposition (i.e., where the patient is actually being sent to treatment)
- Rvw14a: Category of secondary or additional final disposition(s) (i.e., where the patient is actually being sent to treatment):
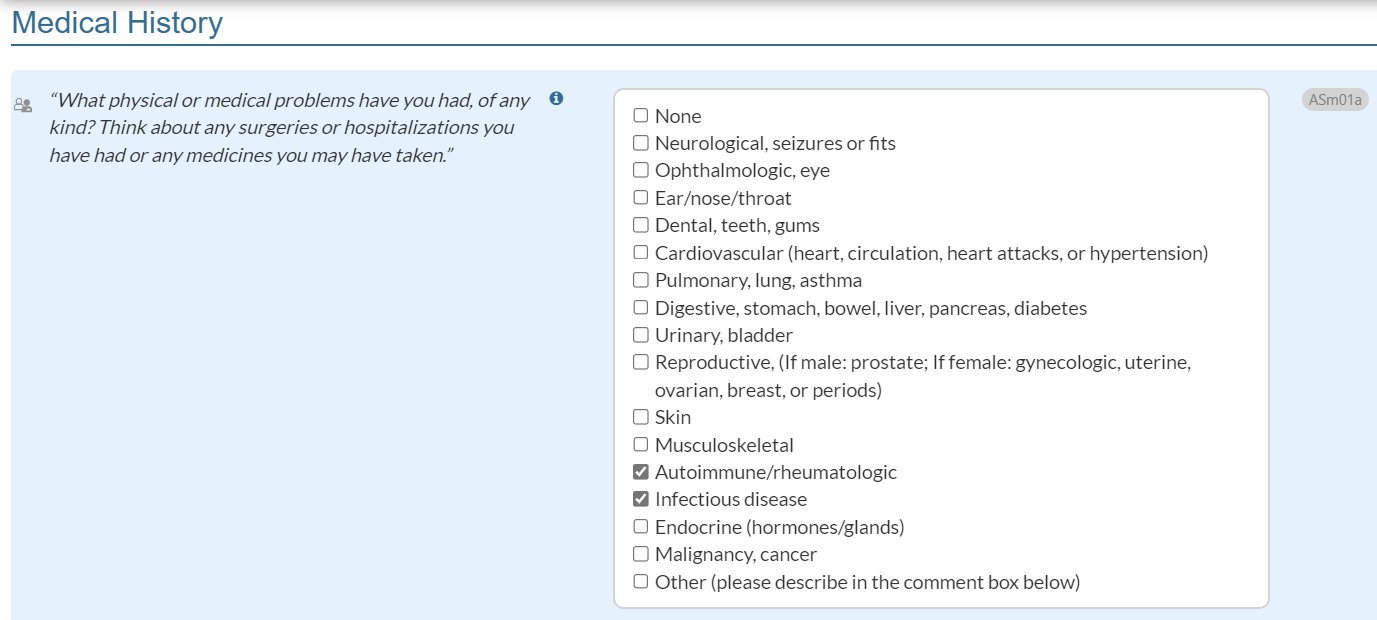
Medical History – Infectious Disease List Revised:
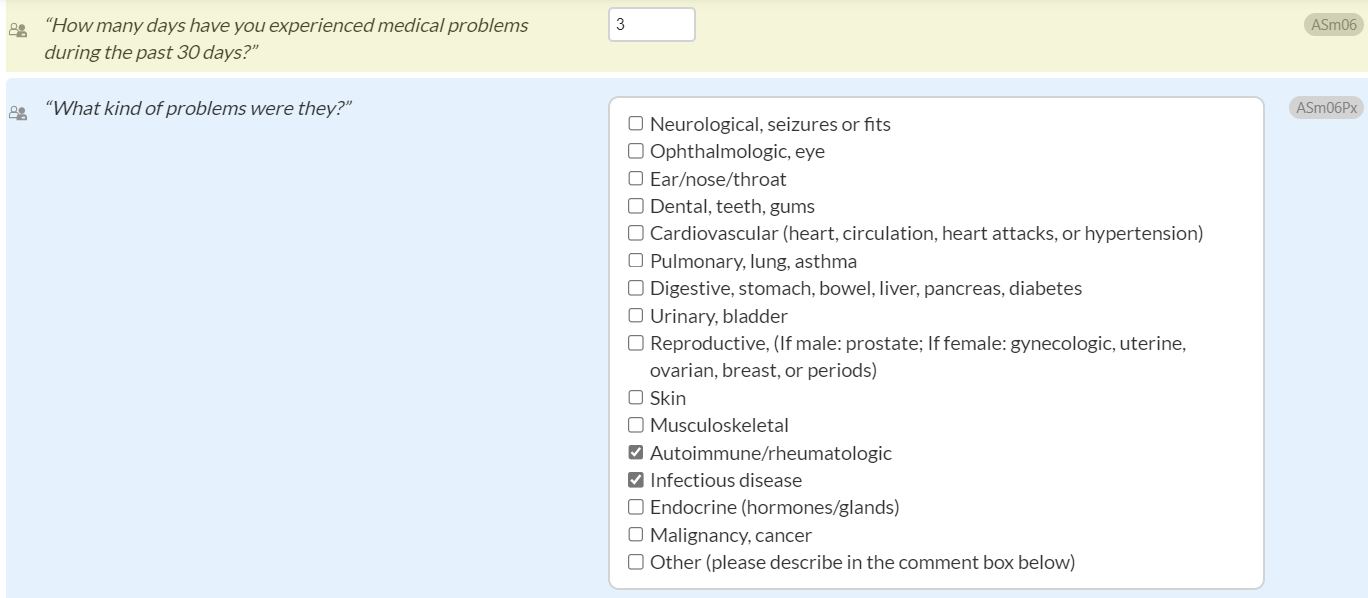
A new response option, “Infectious disease,” is being added to ASm01a (in CONTINUUM) and ASm06Px (in both CONTINUUM and CO-Triage).
The previous response option, “Immune/rheumatologic” for both items will be changed to “Autoimmune/rheumatologic.”
The changes will appear as follows:
For ASm01a (CONTINUUM only): "What physical or medical problems have you had, of any kind? Think about any surgeries or hospitalizations you have had or any medicines you may have taken. "
For ASm06Px (both CONTINUUM and CO-Triage): "What kind of problems were they?"
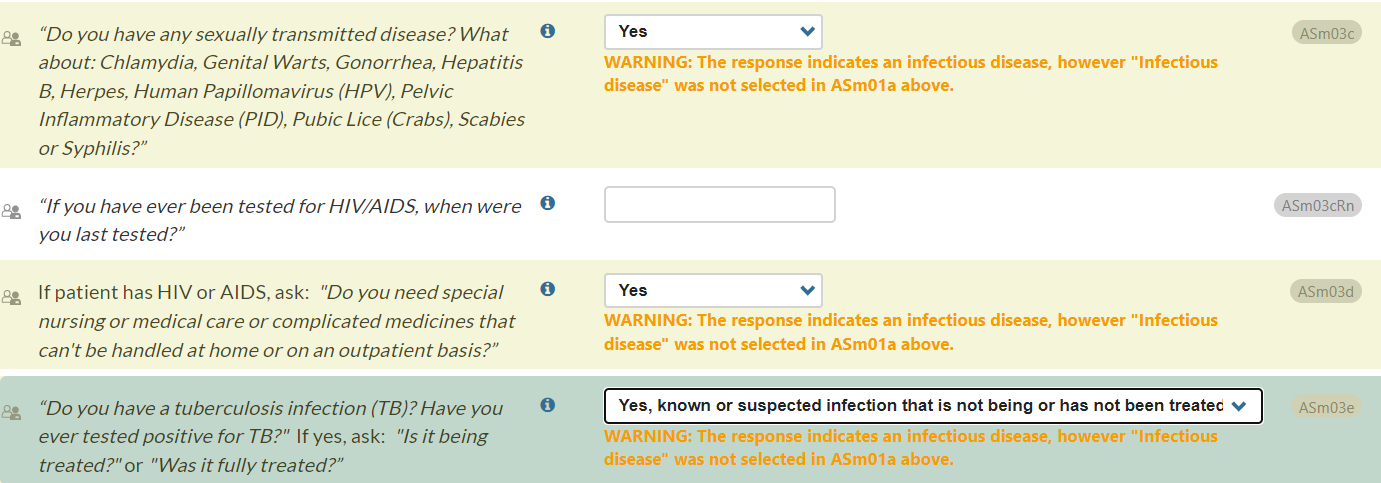
New Warning Message (CONTINUUM only)
To support internal consistency, a warning message will appear if “Infectious disease” is not selected in ASm01a (“What physical or medical problems have you had, of any kind?” ) but “yes” is selected for ASm03c, ASm03d, and/or ASm03e:
- ASm03c: "Do you have any sexually transmitted disease?"
- ASm03d: If patient has HIV or AIDS, ask: "Do you need special nursing or medical care or complicated medicines that can't be handled at home or on an outpatient basis ?"
- ASm03e: "Do you have a tuberculosis infection (TB)? Have you ever tested positive for TB?"
This warning message will appear in amber and will read, “WARNING: The response indicates an infectious disease, however “Infectious disease” was not selected in ASm01a above.”
CONTINUUM Narrative Report
The Narrative Report has now been expanded to include several items that interviewers have found potentially helpful. When the interviewer types in comments for the following items, they will now appear in the Narrative Report:
General Information Section:
- GndrldN: “Please specify your Gender Identity?”
- PrnounsN: “Please specify your Pronouns?”
- ASg19N: If the controlled environment was indicated as "other" above, describe that other controlled environment:
- GenInfoN: Intake Notes
Medical Section:
- ASm01aN: Please provide further detail (e.g., specific diagnoses):
- ASm06PxN: List the other medical problems mentioned:
Employment Section:
- ASe03N: "Describe your profession, trade or skill."
Alcohol & Drug Section:
- ASd25jN: What are the physician's reasons for readmission to Opioid Treatment Service (OTS)?
- SubsUseN: Comments:
Additionally, responses to the following items will be added to the Narrative Report Problem List, Dimension 3 – Emotional, Behavioral, or Cognitive Conditions and Complications:
- ASp01c: “What psychological or emotional problems have you had?”
- ASp01cN: Please provide further detail (e.g., specific diagnoses):
- ASp19j: Given the history and any new information, what active psychiatric diagnoses does the patient seem to have (other than substance use disorder)?
- ASp19jC: Please provide further detail (e.g., specific diagnoses):
- NOTE: This question appears if ASp19j is answered as “Other.”
Release Notes – Version 3.18 (January 10, 2023)
New Response Option for Pronouns and Gender Identity: “Interviewer Declined to Ask”
In both CONTINUUM and CO-Triage, for these two items:
- “What is your Gender Identity?” [GndrId (CONTINUUM only)]
- “What are your Pronouns?” [Prnouns/TPrnouns (CONTINUUM/CO-Triage)]
If “Interviewer declined to ask” is selected: - The Narrative and Summary Reports (CONTINUUM) will output “they/them” where pronouns are used.
- Report headers will only show the preferred name and will not specify pronouns or gender identity.
These changes have been made in response to a user request. This modification will allow interviewers more flexibility when assessing patients who express discomfort about being asked for their pronouns and gender identity.
CONTINUUM Narrative Report
In the CONTINUUM Narrative Report “Identifying Information” section, Gender Identity and Pronouns will now appear underneath the “Name” field, while Race and Ethnicity will be displayed under “DOB.” This change is intended
to facilitate review of demographic information.
Release Notes – Version 3.17 (December 6, 2022)
End User License Agreement (all products):
The end user license agreement has been updated. Specifically, the previous “Indemnification” paragraph was removed and was replaced with a new paragraph titled “Liability: Breach Notification.” CONTINUUM and CO-Triage users will be asked to acknowledge receipt of the updated end user license agreement.
Support Ticket Form (all products):
In both CONTINUUM and CO-Triage, users will now be able to submit a support ticket directly from within the assessment. A “CONTINUUM Support” link will be available in the upper lefthand corner of the screen, next to an envelope icon (please
see orange arrow and box below).
When “CONTINUUM Support” is selected, it will generate a new tab within the browser. The “CONTINUUM Support” tab will have prefilled information including assessment ID, current date, and user’s email address. The user can
also add up to 4 additional email contacts. All email contacts will receive an email confirmation. The user can respond to the support email confirmation and send any additional information or documentation related to the issue.
All inquiries can be entered in the “Description of Issue” field.
CONTINUUM Question Updates:
- Medical History section: To simplify the addition of information about biomedical diagnoses, at the beginning of the Medical History section, we have reduced the two comment boxes down to only one.
- Previously, when interviewers endorsed any biomedical issue in ASm01a (“What physical or medical problems have you had, of any kind?”), comment box ASm01bN appeared (Please describe specifically).
- If interviewers selected “Other” for ASm01a, a second comment box would appear (ASm01aN – List the other medical problems mentioned).
- Now, only ASm01aN [Comment box: Please provide further detail (e.g., specific diagnoses)] will display for any selection in ASm01a (“What physical or medical problems have you had, of any kind?”), except “None.”
- ASm01bN (comment box – Please describe specifically) has been eliminated to reduce redundancy.
- Medical History section: To allow interviewers more flexibility when selecting HIV/TB test dates, we have updated the information text icon for the HIV and TB test questions:
- (ASm03cRn) “If you have ever been tested for HIV/AIDS, when were you last tested?”
- (ASm03eRn) “When were you last tested for TB?”
- The information icon for both items now reads (new text in bold), “Click on arrow to choose time frame. If the patient has only been tested once, enter that date. Estimate if not certain or enter the approximate time frame in the Comments question (MedInfoN) at the bottom of the page.”
- Psychological History section: To assist interviewers in entering information about specific mental health diagnoses, comment box ASp01CN will now appear for any response option selected for item ASp01c (“What psychological or emotional problems have you had?”):
- Previously, ASp01CN (comment box – List the other problems mentioned) appeared only when the interviewer endorsed “Other” for ASp01c.
- Now, ASp01CN will appear for any selection in ASp01c, and will be reworded to read: Please provide further detail (e.g., specific diagnoses).
- If the interviewer selects “Other,” comment box ASp01CN will be a required item; otherwise, it will be optional.
- Psychological Interviewer Rating section: We have also added a new comment box, ASp19jC, underneath item ASp19j [Given the history and any new information, what active psychiatric diagnoses does the patient seem to have (other than substance use disorder)?]
- ASp19jC will read, Please provide further detail (e.g., specific diagnoses).
- If the interviewer selects “Other,” comment box ASp19jC will be a required item; otherwise, it will be optional.
Release Notes – Version 3.16 (October 11, 2022)
CONTINUUM and Co-Triage Question Update:
- Drug and Alcohol Section – Used Substances Subsection: For the SubsUse question, we have reworded response options to clarify that the question applies only to substances currently causing a problem. The response option "Any other substances
(e.g., high-dose caffeine, steroids, etc.)" will now read, "Other problem substances (e.g., high-dose caffeine, steroids, etc.)."
- SubsUse in standard CONTINUUM reads: “Which substances have you had problems with? Think about alcohol or drug use that is currently a problem or could become a problem again. Which substances would you like help with?”
- SubsUse in CONTINUUM RISE reads: "Which substances have you had problems with prior to [your arrest/entering jail or prison/rehab/the halfway house/hospital]? What about since then? Think about alcohol or drug use that is currently a problem or could become a problem again. Which substances would you like help with?"
- We are also updating this SubsUse response option in CO-Triage to align with the change to CONTINUUM.
- In CO-Triage, the response option previously read, “Any other drug of abuse (e.g., high-dose caffeine, steroids, etc.)” but will now read, “Other problem substances (e.g., high-dose caffeine, steroids, etc.).”
CO-Triage Report:
- To clarify whether the interviewer left additional comments for given items, when the interviewer does not enter text in Comment Boxes (for questions DrgInfoN, T5N, T1N, T2N, T3N, T4N, and T6N), the Triage Report will read, “The interviewer
did not have any comments for this section.”
- To enhance language in the Triage Report, when interviewers enter information about more than one medical or psychological problem in the Comment Boxes, the report text now reads:
- Dimension 2 – Medical Conditions and Complications, ASm06PxN: “Other medical problems”
- Dimension 3 – Emotional, Behavioral, or Cognitive Conditions, ASp01cN: “Other psychological or emotional problems”
If the interviewer does not endorse other medical or psychological problems in the instrument, the report will not print these items.
- Dimension 2 – Medical Conditions and Complications, ASm06PxN: “Other medical problems”
Release Notes – Version 3.15 (August 30, 2022)
Narrative Report Updates
- We have improved how CONTINUUM response options result in sentence outputs in the Narrative Report. These changes affect the standard version of CONTINUUM.
- Alcohol & Drug Section, Client Perception of Severity of Alcohol and Drug Problems and Desire for Treatment subsection
- In CONTINUUM, for the question "How troubled or bothered have you been in the past 30 days by the noted alcohol problems?" (ASd23a), when responding "considerably” or "extremely," the Report sentence will read "considerably bothered" or "extremely bothered." (Prior to these updates, the text read "bothered considerably" or "bothered profoundly.")
- In CONTINUUM, for the question "How troubled or bothered have you been in the past 30 days by these drug problems?" (ASd23d), when responding "considerably” or "extremely," the Report sentence will read "considerably bothered" or "extremely bothered." (Prior to these updates, the text read "bothered considerably" or "bothered profoundly.")
- Psychiatric Section, in the Severity of Emotional and Psychological Problems and Desire for Treatment subsection
- Similarly, in CONTINUUM, for the question "How much have you been troubled or bothered by the previously discussed psychological or emotional problems in the past 30 days?" (ASp12), if the interviewer responds "considerably" or "extremely,” the Report sentence will read "considerably bothered" or "extremely bothered." (instead of “bothered considerably" or "bothered profoundly.")
- Alcohol & Drug Section, Client Perception of Severity of Alcohol and Drug Problems and Desire for Treatment subsection
- In CONTINUUM, any language entered by the interviewer in the Comment Box of any section will appear in the Narrative Report with quotations.
- Alcohol & Drug, Employment, Family/Social, Legal, Medical, Psychiatric, and Clinical Summary Notes sections:
- The statement will begin with Comments: followed by the text entered by the interviewer with quotations at the beginning and end of the statement.
- Alcohol & Drug, Employment, Family/Social, Legal, Medical, Psychiatric, and Clinical Summary Notes sections:
- We have streamlined information in the Narrative Report regarding patients’ substance use.
- Alcohol & Drug Section, Lifetime and Past 30-Day Use subsections
- If the patient did not endorse any drug or alcohol use, then the Narrative Report will simply state: “Patient did not endorse any alcohol use” or “Patient did not endorse any drug use.”
Summary Report Updates
- If the patient did not endorse any drug or alcohol use, then the Narrative Report will simply state: “Patient did not endorse any alcohol use” or “Patient did not endorse any drug use.”
- Alcohol & Drug Section, Lifetime and Past 30-Day Use subsections
- Critical Items Section
- Any language entered by the interviewer in the text field for ASf19iN [“Describe the abuse/neglect risk and any resulting actions taken by the interviewer or supervisory staff”] in CONTINUUM has been added to the Summary Report with quotations. In the Summary Report, the statement will begin with, The following critical psychological/psychiatric item(s) were noted in this assessment: The interviewer commented that… followed by the text, as entered by the interviewer, in quotations.
Question Updates
- Any language entered by the interviewer in the text field for ASf19iN [“Describe the abuse/neglect risk and any resulting actions taken by the interviewer or supervisory staff”] in CONTINUUM has been added to the Summary Report with quotations. In the Summary Report, the statement will begin with, The following critical psychological/psychiatric item(s) were noted in this assessment: The interviewer commented that… followed by the text, as entered by the interviewer, in quotations.
- In CONTINUUM’s on-screen interview, Psychological Section
- The language for the header, “Are you worried about having another attack?” (immediately above the block containing ASp04dL) has been changed to “Have you worried about having another attack?” to reflect the past tense orientation of response options for this header (In your lifetime/In the last month/In the last 24 hours).
Response Updates
- The language for the header, “Are you worried about having another attack?” (immediately above the block containing ASp04dL) has been changed to “Have you worried about having another attack?” to reflect the past tense orientation of response options for this header (In your lifetime/In the last month/In the last 24 hours).
- In CONTINUUM’s on-screen interview, Interview Completion Section
- To ensure users obtain accurate information about a patient’s willingness to take new medications as prescribed, response options have been updated for the question (see the change in bold below):
"If any medications are being or will be prescribed, is the patient willing and able to self-administer these with good compliance? (Responding "NOT willing or able to safely self-administer the medication" may escalate the Final Level of Care intensity and/or require Biomedical Enhanced Services (BIO). If unsure, consult a nurse or physician.)" (Post06)
Response options now include:
- NO medications are currently prescribed or planned
- Patient is currently on medication, or medication will be prescribed, and IS willing and able to self-administer with good compliance
- Patient is currently on medication, or medication will be prescribed, but patient is NOT willing or able to safely self-administer the medication
User Interface Updates
- To ensure users obtain accurate information about a patient’s willingness to take new medications as prescribed, response options have been updated for the question (see the change in bold below):
- New on-screen pop-up error messages will increase the accuracy of the patient’s substance use and treatment history.
- CONTINUUM Drug and Alcohol Section/ Additional Addiction and Treatment Items
- If interviewers endorse a substance for:
“Which substance is the major problem?” (ASd14, standard)
or
“Which substance has been the major problem or could become the major problem when you re-enter the community?” (ASd14, RISE)
that does not match any of the substances selected for:
“Which substances have you had problems with?” (SubsUse, standard)
or
“Which substances have you had problems with prior to [your arrest/entering jail or prison/rehab/the halfway house/hospital]?” (SubsUse, RISE),
Then an error message will read, “ERROR: [Substance] was not selected in the Used Substances section. Please select a value that matches one of the responses endorsed in the SubsUse question.”
- If interviewers endorse a substance for:
- CONTINUUM Drug and Alcohol Section/Addiction Treatment History
- If interviewers endorse “Alcohol and Drug” for the type of treatment the patient has received (PrevTrmt),
but then select “0” for, “How many times in your life have you been treated for alcohol use problems?”(ASd18a)
and/or select “0” for, “How many times in your life have you been treated for drug use problems?” (ASd18d)
or
If interviewers endorse “Alcohol only” in PrevTrmt,
but then select “0” for, “How many times in your life have you been treated for alcohol use problems?”(ASd18a)
or
If interviewers endorse “Drug only” in PrevTrmt,
but then select “0” for, “How many times in your life have you been treated for drug use problems?” (ASd18d)
Then they will see an error message that reads, “ERROR: The response must be greater than zero based on your answer to the Question PrevTrmt.”
- If interviewers endorse “Alcohol and Drug” for the type of treatment the patient has received (PrevTrmt),
- CONTINUUM Drug and Alcohol Section/ Additional Addiction and Treatment Items
Co-Triage/Triage Report
- Language entered by the interviewer in Co-Triage comment boxes (DrgInfoN, T5N, T1N, T2N, T3N, T4N, and T6N) will appear in the Triage Report with quotations.
- The statement will begin with Comments: followed by the text entered by the interviewer with quotations at the beginning and end of the statement.
Release Notes – Version 3.14 (July 19, 2022)
The American Society of Addiction Medicine is pleased to announce that version 3.14 of the ASAM CONTINUUM and CO-Triage software has been released. Details about the updates to this software version can be found below and will be posted on ASAM CONTINUUM website. In addition, updates appear in both the standard version of CONTINUUM and the RISE.
User interface
● With opioid overdose deaths rising rapidly, it is critical that naloxone, the overdose reversal medication, is in the hands of anyone who needs it. ASAM CONTINUUM users can support patients' safety by talking to them about naloxone, providing overdose prevention training resources, and ensuring patients and their loved ones have access to the medication. We have implemented the following to remind clinicians to provide education and resources to the patient:
○ Drug and Alcohol Section, Additional Addiction and Treatment Items subsection: A pop-up box with naloxone information will appear if the patient has endorsed opioid use and at least one overdose experience in Question ASd17e, “How many times have you overdosed on drugs or alcohol?”
○ Summary Report: Where applicable, the Critical Items section will state, “[Patient Preferred Name] indicated that [he/she/they] has overdosed on alcohol and/or drugs in the past 24 hours. Suspected recent overdose requires rapid evaluation by physician/psychiatrist or emergency room. If the overdose was due to opioids, please equip the patient and loved ones with naloxone.”
Narrative & Summary Reports
● DSM-5 Diagnosis section, Diagnostic Findings subsection: ICD-10 codes and descriptions have been added to improve usability for treatment planning, coding, and insurance authorization.
○ For patients with substance use disorder diagnoses, reports will now display the following alongside the DSM-5 diagnosis, criteria met, and severity of the substance use disorder:
■ Specific ICD-10-CM diagnosis and billable code, e.g., ICD-10 F10.20 Alcohol dependence
Release Notes – Version 3.13 (June 7, 2022)
In the Drug and Alcohol section:
- In the Opioid Treatment Services sub-section, ASd25j has been updated to:
Does the program physician or authorized healthcare professional judge Opioid Treatment Program (OTP) readmission to be clinically appropriate?
The added phrase (in bold) accounts for situations in which healthcare professionals other than physicians make decisions regarding OTP admissions.
The response options for this item now include "Yes/No/Not applicable."
New help/hint text for this item reads, “Choose not applicable if no program healthcare professional is currently available to make this assessment. An authorized healthcare professional can include an advanced-practice nurse, physician assistant, or advanced-practice pharmacist.”
- Consistency checks have been added to the Alcohol Use sub-section to improve data quality. Now, error messages will appear, alerting the user to data discrepancies in the following scenarios:
- If the number of days entered for the patient's use of alcohol to intoxication in the past 30 days exceeds the number of days entered for any alcohol use in the past 30 days;
- If the value entered for lifetime duration of alcohol use to intoxication exceeds the value entered for lifetime duration of any alcohol use;
- If the value entered for the patient's last use of alcohol to intoxication is incompatible with the value entered for the patient's frequency of alcohol use to intoxication in the past 30 days (e.g., the patient's last use of alcohol to intoxication was 25 days ago, but the interviewer reports that the patient used alcohol to intoxication on 7 of the past 30 days).
In the Medical History section:
- We have added two new items that enable clinicians to enter dates for the patient's latest tests for HIV/AIDS (ASm03cRn) and tuberculosis (ASm03eRn), if applicable. Test dates will now appear in the Narrative Report Problem List.
In the Summary and Narrative Reports:
- For patients with a history of suicide attempts, the Summary Report Critical Items section and the Narrative Report Problem List will display the patient's most recent suicide attempt (e.g., past 24 hours, past month) to enhance visibility and support treatment planning.
- Additionally, any past 24-hour suicide attempt will be noted in the Narrative Report, Psychiatric Section, at the start of the second paragraph (titled “Severity of Emotional and Psychological Problems and Desire for Treatment”).
Expand all
Collapse all
User Release - Previous Updates
Version 3.9 Release (December 21th 2021)
CO-TRIAGE Update:
The Review Section of CO-TRIAGE will now display as “100% complete” when all questions in this section are answered. Section completion progress can be tracked through the percentage listed on the navigation panel. This update was initiated to encourage users to report the Final Level of Care Placement and final disposition data.
ASAM CONTINUUM Updates:
In the Medical History section, if the user indicates that a patient is experiencing signs or symptoms that may require a medically monitored Level of Care (e.g., fever of 102 degrees or more, unsteadiness on feet or walking problems, HIV/AIDS, toxic psychosis, etc.), but the clinician does not endorse that the patient needs inpatient medical monitoring in ASm09 (How would you rate the patient’s need for medical treatment?), a warning message will now appear to alert the clinician to consider a medically monitored Level of Care. The following message will appear in orange: “WARNING: The response to earlier items suggests that the patient has immediate possible medical problems (e.g., uncertain disulfiram reaction, toxic psychosis, delirium tremens, overdose, significant head trauma, seizure(s), extreme fever, neurological condition, liver disease, acute pancreatitis, or another condition which could be severely and immediately dangerous with continued substance use). These are problems that may require further evaluation, on-site medical monitoring, and immediate changes in the treatment plan. Did you mean to select this option?”
In the General Information section, ASg19 (Have you been in a controlled environment in the past 30 days?) now appears on-screen above ASg14 ( “How long have you resided in your current living situation?”). If the user selects a controlled environment in ASg19, they will be prompted to complete a RISE assessment. If they elect to switch to the RISE, ASg14 will change to the RISE wording. This prevents users from having to answer ASg14 twice – first in the standard CONTINUUM, then again in RISE.
Report Updates:
To increase the utility of the Narrative Report for users, we have added two new sections, i: (1) a Final Level of Care Recommendation and (2) an Actual Level of Care
Question & Response Updates:
- In the General Information Section if patients report that they have been in any controlled environment in the last 30 days (e.g., jail or prison)(ASg19), then the response to item ASl01z in the Legal Section, which asks if the client has been arrested or charged for any reason, should be “Yes.”
- If “No,” is selected, an error message will now appear stating:
“ERROR: The response cannot be “No” as the patient reported on ASg19 that they have been or are currently in jail or prison.”
- If “No,” is selected, an error message will now appear stating:
- To further promote gender identity inclusivity, questions related to pregnancy in the Medical History Section (e.g., ASm03a) will display regardless of the patient’s reported gender. These updates affect questions in CONTINUUM and CO-Triage.
- In the Medical History Section, the question “Do you have any unsteadiness on your feet or problems with walking or balance, such that you could easily fall or have trouble getting around or using stairs?” (ASm06i) has been updated. The revised question includes new clinical guidance and response option details. These language improvements are designed to help increase the accuracy of data entry.
- In the Drug & Alcohol and Medical History Sections, the abbreviation “(DTs)”, for “Delirium Tremens”, has been added to questions ASd17a and ASm01.
- Drug & Alcohol Section: ASd17a, “How many times have you had alcohol-related Delirium Tremens (DTs)”?
- Medical History Section: ASm01, “How many times in your life have you been hospitalized for medical problems?" (Include hospitalizations for overdoses and Delirium Tremens (DTs), but exclude withdrawal management hospitalizations)”
- In the Medical History Section, question ASm06g (“Has the patient had Delirium Tremens (DTs)…?”) now has an Info/Help icon that states:
- “Delirium tremens (DTs), technically called “alcohol withdrawal delirium,” is a sign of severe alcohol withdrawal. Its symptoms include agitation, altered mental status (global confusion and/or disorientation), visual and/or auditory hallucinations, fever, high blood pressure, sweating, and fast heartbeat.”
- “Delirium tremens (DTs), technically called “alcohol withdrawal delirium,” is a sign of severe alcohol withdrawal. Its symptoms include agitation, altered mental status (global confusion and/or disorientation), visual and/or auditory hallucinations, fever, high blood pressure, sweating, and fast heartbeat.”
- In the Interview Completion Section, a question header has been added for questions ASm06r and ASm06s:
- ASm06r: “Does the patient exhibit any symptoms that would be considered life-threatening AND are related to alcohol or drug use?” (Immediate and life-threatening symptoms should result in a direct transfer to a medical hospital.)
- Asm06s: “Would the patient’s current substance use, or resumption of substance use, be likely to cause a severely dangerous exacerbation of a medical condition?” (Important: This question asks whether a specific, severely dangerous exacerbation is likely to occur TODAY if the patient continues to use. Do not consider theoretical, generic, future risks.)
- The header now states “For the following two questions, please note responding “Yes” could escalate the Final Level of Care intensity…” This warns the interviewer that responding “Yes” to these questions could escalate the Final Level of Care intensity and/or require Biomedical Enhanced Services (BIO).
- In the Interview Completion Section, the question “If any medications are being or will be prescribed, is the patient willing and able to self-administer these with good compliance?” (Post06) has been updated.
Now, if Post06 is answered with “No – patient is currently on medication, or medication will be prescribed, but patient is NOT willing or able to safely self-administer the medication”, but it was indicated in the Medical History and/or Psychological History sections that the patient does not have medical problems and is not taking medications, a pop-up alert will appear with this message: “You had previously indicated that the patient does not have medical problems and is not on medications for physical or psychological problems. Did you mean to select this response?” Response options include “Yes” or “No”.
Narrative and Summary Report Updates:
- Pregnancy & Gender Identity
Language improvements have been implemented to promote gender identity inclusivity when a patient reports a current pregnancy. - Patient’s Preferred Pronouns
The sentence logic has been updated to more reliably use the patient’s preferred pronouns. - (CONTINUUM RISE only) The Problem List in the Narrative Report has been updated with the most current 8-character codes. Language in the Item Statements have been enhanced with rephrasing appropriate for RISE patient populations.
Summary & Narrative Report Updates:
- The Summary and Narrative Reports now specify the type of assessment conducted – (standard) CONTINUUM or RISE (Re-entry Interview Script Enhancement). The cover pages of both reports have been updated with either “ASAM CONTINUUM Assessment” or “ASAM RISE Assessment (Re-entry Interview Script Enhancement)” in multiple locations.
- The Legal Section of the Narrative Report has been edited for clarity. This section will now list legal charges the patient has incurred (e.g., “[Patient] has been charged with assault on one occasion”) but will omit statements about legal charges the patient has not incurred (e.g., “[Patient] has never been charged with homicide”).
- The “Diagnostic Suggestions” section in the Summary Report has been renamed to “Diagnostic Findings” and has been added to the Narrative Report for more comprehensive clinical documentation.
ASAM CO-Triage Updates:
- The CO-Triage interface has been updated to improve consistency in layout and wording.
- References to other assessment questions have been removed from TASm03b (pregnancy status) and ASd24w (concerns about pursuing treatment) to streamline the user experience.
- ASm09 (severity of physical health problems), which references the user’s response to ASm06Px (physical health status), has been updated with the correct item label for ASm06Px.
- The two-person silhouette icon has been added to question ASm09, and the question has been italicized, to clarify that it should be asked directly to the patient.
Question Updates:
In the Drug and Alcohol section, updates ensure alignment between responses regarding the patient’s last use of a substance and past 30-day frequency of use. An error message will now display if the interviewer has indicated a need for alcohol use treatment (ASd25a) when the patient did not endorse alcohol use. A similar error message will display if the interviewer has indicated a need for drug use treatment (ASd25d) when the patient did not endorse any (non-alcohol) drug use.
Question Updates (CONTINUUM RISE only):
Help/hint text in the blue info icons now better reflects the future-oriented, re-entry focus of the following RISE questions:
ASf04, “When you re-enter the community, what will your living arrangements be?“
ASf05, “How long did you live in these arrangements before entering the controlled environment?” (If with parents or family, only count since age18)”
ASf19j, “Upon re-entering the community, what is the likelihood that you could be hurt or victimized by another?”
ASf19d, “Upon re-entry, will you be in close contact with anyone you have abused or neglected?”
ASf19e, “Is that neglect or abuse likely to occur during your substance use?”
ASf19i, “Upon re-entering the community, what is the likelihood that you could cause harm to or neglect others?”
ASf19f, “Is it likely that family neglect/abuse will worsen without care at a level greater than Level 2?”
ASf19k, “Is the risk of harm only a problem during alcohol or drug intoxication?”
ASf19l, “Upon re-entering the community, will you be able to locate and get yourself to community resources safely?”
Narrative Report Updates:
We have clarified statements regarding the amount of time a patient has spent in a correctional facility.
Free text entered in the Medical Section of the assessment now outputs more clearly in the Medical History subsection of the Narrative Report.
Output in the Interviewer Impressions and Recommendations – Alcohol and Drugs subsection regarding primary substance now reads, “Currently, {name of substance} is/are his/her/their most significant substance use problem.”
Statements in the Alcohol & Drug Section regarding lifetime and past 30-day use have been separated into different paragraphs to improve readability.
Sentences stating which substances are not problematic for the patient will no longer appear in the Narrative Report output.
Various terms have been updated to reflect more current terminology, e.g., “opiates” has been replaced throughout with “opioids.”
RISE output regarding the patient’s probation/parole status has been clarified to state that the patient will be on probation/parole upon re-entry into the community (if the interviewer endorses this in the assessment).
Summary Report Updates:
We have clarified the Final Level of Care Recommendations section. Level of Care recommendations are now more clearly presented as a package of services to be combined as needed. Withdrawal Management, Opioid Treatment Services (OTS), and Opioid Treatment Program (OTP) recommendations, if applicable, are now listed before any Level of Care recommendations that should be considered in order to address patients’ needs in Dimensions 2 through 6.
RISE Updates
- Clinicians administering RISE assessments can now change their selection for controlled environment type (Jail, Prison, etc.) prior to submission without having to create a new assessment.
Response Updates
- In the Psychological History Section, the response options regarding the level of severity for “Trouble controlling violent behavior” (ASp07L, ASp07M, ASp07D) and “Serious thoughts of suicide” (ASp08L, ASp08M, ASp08D) have been updated along with the blue circle “i”nformation icon guidance for enhanced clinical accuracy.
Narrative Report Updates
- Report output clarity has been improved for “Not sure or possibly” responses to the question, “Does the patient currently have symptoms or signs of intoxication?” (ASm06a). The output, which appears in the Alcohol & Drug Section of the Narrative Report, now reads, “It is not clear if the patient has symptoms or signs of intoxication.”
- Output in the Interviewer Impressions and Recommendations – Alcohol and Drugs sub-section has been updated. It previously read, “It is my belief that [e.g., barbiturates] are [his] most significant substance use problem.” The updated sentence reads, “Currently, [barbiturates] are [his] most significant substance use problem.”
- The Narrative Report has been streamlined to improve readability: When a patient has never used a substance and therefore answers no questions about alcohol treatment history, we have eliminated repetitive statements about all the drug categories that have never been used from the Alcohol & Drug Section.
Summary Report Updates
- Language has been updated and streamlined in the Final Level of Care Recommendations Specific changes include:
- Dimension 1 Withdrawal Management (WM) and Opioid Treatment Services/Opioid Treatment Program (OTS/OTP) recommendations now appear first, before other Levels of Care that may also be recommended to address patients’ needs in Dimensions 2 through 6.
- If the patient did not meet criteria for BIO, but the interviewer selected “Not sure or possibly” for certain medical assessment questions, there will be a recommendation for additional medical evaluation.
- Recommendations for OTS and OTP have been edited to improve clarity and avoid redundancy.
Question and Response Updates:
- In CONTINUUM RISE, guidance has been added to question ASm06s – “Would the patient’s current substance use, or resumption of substance use, be likely to cause a severely dangerous exacerbation of a medical condition?” – which reads: “(Important: This question asks whether a specific, severely dangerous exacerbation is likely to occur TODAY if the patient continues to use. Do not consider theoretical, generic, future risks. Responding “Yes” or “Not sure/possibly” will escalate the Final Level of Care intensity and/or require Biomedical Enhanced Services (BIO). If unsure, consult a nurse or physician.)”
- To promote destigmatizing language, the response option “Any other drug of abuse…” in the Used Substances list (Drug and Alcohol section) has been rephrased as, “Any other substances (e.g., high-dose caffeine, steroids, etc.)”
- We have corrected a bug that was causing responses of “No” or “None” to pre-populate for some items in the Medical History section and Psychological History subsection.
- The “Legal Information” and “Family and Social History” sections now correctly show “100%” under “% Complete” once all required questions have been answered.
Summary Report Updates:
- The Critical Items Section of the Summary Report previously included statements affirming that the patient reported certain medical problems (e.g., liver problems) if the clinician responded either “Yes” or “Not sure or possibly” to questions asking whether those medical problems were present. The Summary Report logic has been corrected so that these statements only populate if the response is “Yes.”
- Addition of CO-Triage report output for cases in which the patient meets Level 1 or Level 2 criteria, but needs assistance with housing/shelter, transportation, or ambulation/mobility in order to attend outpatient treatment. For Level 1, the output will now read: “This individual has met the provisional requirements for Level 1 – Outpatient Services. To succeed at this level, the patient may need assistance with shelter, transportation, or other help with mobility to consistently attend treatment.” The output for level 2 reads the same, substituting Level 2 for Level 1.
ASAM CONTINUUM Narrative Report Updates:
- The Psychiatric Section of the Narrative Report now describes more precisely the intensity of patients’ emotional, behavioral or cognitive symptoms during the clinical interview. For instance, if the interviewer endorses that the patient presented with slight symptoms of depression, the Narrative Report will feature the following statement: “The patient was slightly depressed at the time of the interview.” If the interviewer endorses that the patient presented with moderate symptoms of anxiety, the statement will read: “The patient was moderately anxious at the time of the interview.” These updates appear in both RISE and standard versions of CONTINUUM.
Question and Report Updates:
- In the Interview Completion Section, the Clinical Summary Notes field (IntCompN) now includes guidance on the type of patient information to report in this field. It now reads, “Clinical Summary Notes (Any additional information and/or an overall formulation of the patient’s status, needs, strengths, etc.)”. This information now can be viewed in the Narrative Report under “Psychiatric Section – Interviewer Comments.” This update appears in both the standard version of CONTINUUM and the RISE.
- In the Alcohol & Drug Section, refinements have been made to questions regarding prescribed substances:
- In each of the alcohol/other drug subsections, questions about whether the patient responded to efforts to maintain prescribed substances at prescribed doses (e.g., ASd03Qa) have been reworded to ask whether the prescribing provider tried to help the patient maintain the prescribed dose and frequency. These questions are now asked directly to the patient.
- In the Heroin and Fentanyl Use subsection, a new question asks whether the patient used heroin, fentanyl, or both (ASd03U).
If fentanyl is endorsed, another new question (Asd03X) asks whether fentanyl was prescribed.
If fentanyl was prescribed, question ASd03Q now asks whether the patient took fentanyl at the prescribed dose (or “Less” or “More”).
If the response is “More,” new question ASd03Qa asks whether the prescribing provider tried to help the patient maintain the dose and frequency that were prescribed.
- In the Nicotine and Tobacco Use subsection, a new question has been added to determine whether the patient used tobacco, other nicotine products, or both (ASd13aU).
If use of other nicotine products is endorsed, question ASd13aX now asks whether nicotine products (no longer “tobacco and other nicotine products”) were prescribed, and ASd13aQ now asks whether nicotine products (no longer “tobacco and other nicotine products”) were taken at the prescribed dose. New info icon guidance is available to assist the interviewer on these updates. The Narrative Report Problem List now presents responses to the new and re-worded questions that indicate problem use.
- In the Alcohol & Drug Section substance-specific subsections, an error has been corrected in the response options under the heading, “Have you given up or cut back in any important activities because of the drug you previously described?” Previously, the response option “Job” was included under this header, but it has now been moved to the correct header below, which reads, “Have you continued your use of the previously described drug despite having problems again and again with…”?
Response Updates:
- In the Family and Social History section, the numerical response format for question ASf09 (“How many close friends do you have?” in standard CONTINUUM; “How many close friends will you have when you re-enter the community?” in RISE) has changed from a text field to a dropdown menu. The response options range from “0” to “5 or more”. In RISE, ASf09 now includes “Unknown” as a possible response option for this question.
These enhancements were made to increase accuracy of responses.
Co-Triage Response Update
- Users can now select “unable to assess” for ASm0: When this response option is selected, the report output will read: “The patient was unable to assess the need for medical/nursing care or physical rehabilitation. Further assessment by a medical professional is recommended.”
- Users will also see new report output for patients who meet Level 1 but may need additional case management for transportation/shelter/mobility so they can access that Level of Care.
Important Clinical Updates:
- Religion & Ethnicity: Patient profile information has been updated to accurately reflect patients’ religion and ethnicity data. Previously, these data were not being passed from the EHR into CONTINUUM in a consistent manner, causing differences in the ways patients’ religion and ethnicity appeared in the ASAM CONTINUUM Narrative Report versus the EHR. A consistency check has been added to ensure alignment. The Narrative Report sentence logic also has been restructured to state the patient’s ethnicity more clearly, and the sentence logic regarding race has been omitted, as CONTINUUM does not collect race data separately from ethnicity data.
- Question and Report Updates:
- Substance Use Pattern: In the Drug & Alcohol section, after selecting the substance(s) used, the question “When you usually [drink, drank, use(d)] alcohol, what [is, was] the specific [brand, type, name] and how much would you typically [drink/use] per day?” has been added as a free text field to the top of the sub-section collecting information about the patient’s use history and patterns.
- DSM-V Criteria List: The Narrative and Summary Reports have been enhanced to list all DSM-V diagnostic criteria that have been met for each substance. Reports also include the free text response about the patient’s use preferences and patterns for each substance. This information now appears in the Summary Report in the DSM-5 Diagnosis section, and in the Narrative Report at the beginning of the Problem List.
- Final Disposition Data: Data from the post-interview Review Section have been added to the CONTINUUM Narrative Report and the CO-Triage Report so users can easily see information about final disposition.
User Interface Updates:
- Version Numbering: The current software version number has been added underneath the main ASAM CONTINUUM logo in the top left-hand side of the screen.
CO-Triage Interview:
- Interview Type: A question has been added to CO-Triage to indicate how the interview was conducted (e.g., in person, by telehealth video, etc). The CO-Triage Report will now show this information.
- Ecstasy – Important Clinical Update: Responses to the question “Which substances are you having problems with that you would like help with?” have been updated. Ecstasy is now listed with other hallucinogens within the selection “PCP, ecstasy, or other hallucinogens.” This is an update as it was previously listed within the selection “Stimulants other than cocaine (e.g. amphetamines, bath salts, etc.).”
Release Notes – Version 3.12 (April 26, 2022)
CONTINUUM Updates for both Standard and RISE:
Demographic fields have been added to CONTINUUM to capture more accurate patient information, make interviews more patient-centered, and enhance the therapeutic alliance.
- The General Information section will now include:
- Preferred Name (NamePref): The preferred name is what the person identifies with and prefers to be called. It may differ from their legal name. The preferred name will be displayed above the name as stored in the EHR to help remind the clinician to use the patient’s preferred name.
- Gender Identity (GndrId): Note that cisgender means the person identifies as their assigned sex at birth. This is clarified in an information icon attached to the new gender identity question.
- Pronouns (Prnouns): These will be displayed at the top of the assessment and will be used in the reports. If “Other” is selected, the Interviewer can specify the patient’s preferred pronoun. In this case, “they/them” pronouns will populate the reports and “Other” will be displayed in the assessment header (top of the screen).
- A pop-up at the beginning of the assessment will display the values for religion, race, and ethnicity as found in the EHR. This pop-up will give the clinician a chance to check these answers with the patient and correct any errors, so they do not populate incorrectly in the CONTINUUM Narrative and Summary reports.
- Religion: More options have been added according to the data collection standards of the U.S. Centers for Disease Control (CDC)
- Race: More than one race can now be selected at a time
- Ethnicity: Options are based on CDC data collection standards
- Narrative Reports will now display preferred name, gender identity, and pronouns in the “Identifying Information” section on the first page.
- Narrative and Summary Report output will reflect the new demographic information.
Note that responses to demographic items in the CONTINUUM assessment, and updates to demographic information in the pop-up, will NOT change what is in the EHR. If there is a discrepancy between EHR data and the patient’s responses to these demographic items in the CONTINUUM interview, please inform your EHR accordingly.
CO-Triage Updates:
Demographic fields have been added to CO-Triage to capture more accurate patient information, make interviews more patient-centered, and enhance the therapeutic alliance. These include the Preferred Name and Pronouns, as described above.
- Preferred Name (NamePref) - The preferred name is what the person identifies with and prefers to be called. It may differ from their legal name. The preferred name will be displayed above their EHR-derived name in the header (top of the screen) to help remind the clinician to use the patient’s preferred name.
- Pronouns (Prnouns) – These will be displayed in the assessment header (top of the screen). Clinicians can specify the pronoun if “other” is chosen.
- The CO-Triage report will now display the patient’s preferred name and pronouns at the top of the first page.
Note: Responses to demographic items in the CO-Triage assessment will NOT change what is in the EHR. If there is a discrepancy between EHR data and the patient’s responses to these demographic items, please inform your EHR accordingly.
Narrative & Summary Report Updates:
- To assist clinicians with placement and treatment planning, we have created a new section of the Narrative Report that displays the GAF Score in a chart format. Users will now be able to view the Score Range, Patient Score, and a Description of the level of functioning. The chart is located below the Addiction Severity Index Composite Scores sub-section.
- Language enhancements have been made in the Client Perception of Severity of Family and Social Problems and Desire for Treatment sub-section of the Family/Social Section in the RISE Narrative Report to better convey patient responses to the following questions:
- ASf10b (“In the first 30 days after you re-enter the community, on how many days do you think you will have serious conflicts with your family?”)
- ASf10a ("In the first 30 days after you re-enter the community, on how many days do you think you will have serious conflicts with other people (exclude family)?")
- ASf19g (“Think about the person(s) with whom you’ll have contact upon re-entry. Who will you have contact with and who will be most important to you?")
- ASf20 ("Upon re-entering the community, how troubled or bothered do you think you will be by family problems?")
- ASf21 ("Upon re-entering the community, how troubled or bothered do you think you will be by social problems?")
End-User License Agreement Language Update:
- The indemnification clause in the End-User License Agreement (EULA) has been updated to add the language “to the extent permissible by law and to the extent an appropriation has been provided for this contract.” This update keeps ASAM in compliance with state and federal requirements.
Release Notes – Version 3.11 (March 15, 2022)
Audit Report Update:
- Users can now access Audit Reports for their organizations viewable by activity type and timestamp. Reports will consist of data from submitted assessments in both CONTINUUM and CO-Triage.
Question Update:
- In the Drug and Alcohol section, Used Substances sub-section, a Comment Box has been added below the question asking “What substances have you had problems with?” (SubsUse) to help clinicians record details shared by the patient about substance use patterns, preferences, and history.
Narrative & Summary Reports:
- In the Narrative Report, General Information section, we have added content regarding the patient’s current living situation (ASg14) to provide clinicians with further details for treatment planning.
Release Notes – Version 3.10 (February 1, 2022)
Question Updates:
- Throughout CONTINUUM and CO-Triage, we have simplified the use of fonts to improve the clarity of the user interface.
- In the Psychological Interviewer Rating section of CONTINUUM, ASAM has added a hyperlink to the Global Assessment of Function (GAF) scoring scale, thanks to the requests of end-users. Now, interviewers will have the GAF Scoring Scale readily available during all assessments.
User Interface Updates:
- Warning messages will now replace popups where possible to provide data entry guidance while keeping all parts of the screen visible. Additionally, error messages will appear in red to better distinguish them from warning messages, which will appear in orange – as in a traffic light color scheme.
Narrative Report Updates:
- The question, “With whom do you spend most of your free time?” (ASf07) has been added to the Problem List on the Narrative Report.
- The Lifetime and Recent Alcohol/Drug Use sub-sections of the Alcohol & Drug Section are now separated into paragraphs for improved clinician navigation and identification of patient needs. This section now includes Lifetime Alcohol Use, Past 30-Day Alcohol Use, Lifetime Drug Use, and Past 30-Day Drug Use. Other paragraphs in the section have not changed.
Version 3.1.2 updates ASAM CONTINUUM to improve consistency within the assessment and improve the Narrative and Summary Reports. Details regarding the release can be found below.
Bug Fixes within the Assessment:
- The internal error checking previously checked “How long was your last period of voluntary abstinence…?” (ASd15) against “When was your last use of [substance]” (ASd##R). When the two response values were not equal, the system triggered a pop-up error message. This update now allows for the possibility that the patient may have stopped using involuntarily prior to the assessment (e.g., due to incarceration), so that there can be different values for ASd15 and ASd##R.
Narrative Report Bug Fixes:
- Medical Section: On question ASm09, “How would you rate the patient’s need for medical treatment?”, when the interviewer selected “1” or “2” (“minimal health issues”), a logic error caused the Narrative Report to describe patients’ medical problems as “moderately severe” . This has been corrected.
- Legal Section: The Narrative Report previously did not display all arrests/charges that were endorsed. This has been corrected for both standard and RISE versions of ASAM CONTINUUM.
- Psychiatric Section: The “Serious Emotional and Psychological Problems – Lifetime” subsection of the Psychiatric Section incorrectly reported the history of depression and suicidal ideation for some patients. This was due to faulty sentence string logic, which has been corrected.
- Numerous other minor language and reporting improvements have been made as well.
Summary Report Bug Fix:
- Final Level of Care Recommendations Section: Recommendations for some Co-Occurring Enhanced Levels of Care previously stated that the patient “met the diagnostic criteria for a mental disorder.” Since ASAM CONTINUUM currently calculates DSM-5 diagnoses for substance use disorders but not other psychiatric disorders, this sentence has been deleted.
RISE-Only Fixes:
- ASAM CONTINUUM RISE (i.e., the Re-entry Interview for patients leaving a controlled environment): The Narrative Report has undergone multiple changes to the time frame of assessment questions. For example, “How many close friends do you have?” is now asked in the future tense in RISE: “How many close friends will you have when you re-enter the community?”. A few other examples of the many specific changes include:
- General Information Section: A CONTINUUM sentence stating how long the patient has lived at their current address has been rephrased in RISE to: “Before entering the controlled environment, [patient] had lived at [his/her/their] prior address for [time period].”
- Employment and Support Section: A CONTINUUM statement of past 30-day income has been rephrased in RISE, e.g.: “In the past 30 days, [patient] has made [$] income. This includes work while in the controlled environment. In the first 30 days after re-entering the community, [patient] expects to be paid for working on [#] days.”
- Employment and Support Section: A CONTINUUM statement as to whether the patient has a driver’s license is now rephrased for in RISE: “When [patient] re-enters the community, [he/she/they] will [have/not have] a valid driver’s license.”
- Alcohol and Drug Section: CONTINUUM data such as past 30-day alcohol/drug use history are now rephrased in RISE, e.g., “[He/she/they] reports experiencing alcohol problems on [#] of the last 30 days during the time period when [he/she/they] most commonly used In the 30 days after [he/she/they] re-enter[s] the community, [he/she/they] expects to be [moderately/slightly/etc.] bothered by these problems.”
ASAM is pleased to announce that Version 3.1 of the ASAM CONTINUUM and CO-Triage software will go live on Thursday, August 27th. Preliminary details regarding the upcoming release can be found below.
Important Clinical Updates in Version 3.1:
- Updating: Revision of OTP (Opioid Treatment Program, i.e., federally licensed methadone maintenance programs) questions and decision logic to align with current federal OTP regulations
- Streamlining: Removal of the “Opioids and a Controlled Environment” CONTINUUM section
- Improved Consistency: Patients who qualify for OTP will also receive a Final Level of Care recommendation for OTS (Opioid Treatment Services, including OBOT)
- Improved On-screen Tracking: The question numbering system has been revised throughout CONTINUUM and CO-Triage to improve intuitiveness and long-term utility.
Changes to the OTP and OTS assessment questions and decision logic include:
- Removal of tolerance and withdrawal requirements: Current symptoms of tolerance and withdrawal are no longer required for OTP admission under federal regulations. Instead, the algorithm requires that a patient must have met DSM-5 criteria for opioid use disorder for at least one year prior to admission.
- Exception for formerly incarcerated patients: The General Information section now asks whether the patient has been released from a penal institution in the past six months (rather than the past 14 days), or will soon be released; and the “Opioids and a Controlled Environment” section has been removed since tolerance and withdrawal symptoms are no longer required for OTP admission.
- Exception for pregnant patients: Recent opioid use is no longer required for OTP admission. The decision logic has been updated to reflect current requirements of confirmed pregnancy and an active DSM-5 diagnosis of opioid use disorder.
- Exception for previously treated patients: OTP readmissions no longer include the requirement that a patient must have previously been prescribed an opioid withdrawal protocol. Instead, patients can simply have received treatment from an OTP within the past two years. CONTINUUM’s decision logic has been updated to reflect this change.
- The decision logic has also been updated to ensure that since OTP is a subset of Opioid Treatment Services, all patients who meet OTP will also receive a Final Level of Care recommendation for OTS.
Updates to the On-screen Numbering System:
Version 3.1 improves the on-screen numbering system throughout the assessment for questions in both CONTINUUM and CO-Triage. The new system will facilitate communication between assessment users and technical support staff even if the question sequence changes over time. It also will identify the source of questions whose content or format is drawn from other validated instruments (e.g., Addiction Severity Index, CIWA – Clinical Institute Withdrawal Assessment for Alcohol, CINA – Clinical Institute Narcotic Assessment).
ASAM has developed a guidance video to provide a detailed explanation of the changes you will see with this release.
Other recent guidance videos can also be found below.
- Demonstration of the streamlining updates in Version 3.0
- Demonstration of the response item and guidance updates in Version 3.0
CONTINUUM Re-Entry Interview Script Enhancement (RISE)
Version 3.1 makes further improvements to the RISE, for patients who are in a controlled environment and will soon re-enter the community:
- Increased sensitivity: Addition of questions about alcohol- or drug-related problems experienced in the past 12 months and past 30 days (even if an incarcerated patient reports no use in those time periods). Such problems could include increased craving, for example, even in the absence of use – or in anticipation of release.
- Similarly, RISE now includes questions about how much patient has spent on alcohol or drugs (including nicotine) in the past 30 days, (even if the patient reports no use in the past 30 days) – because these items may be used as currency in an incarcerated setting or patient may have purchased items with the intent to use at a later time.
- Streamlining: Removal of the question, “Is the patient currently residing in Level 3.1 care?” since Level 3.1 is generally not available to patients who are currently incarcerated.
Version 3.0 Details:
Important Clinical Updates:
Version 3.0 makes significant updates to the tool:
- clinical changes to the question structure
- additional guidance and help messaging for delivering the interview
- expanded response items
Version 3.0 integrates greater efficiency in navigating through the interview by streamlining the assessment and eliminating questions that are not applicable to the patient in response to a triggering item. These revisions are applicable in the Medical History; Drug and Alcohol; Legal Information; Family and Social History; and Psychological sections. This release also expands and improves available response options for a number of questions.
A detailed list of item changes and guidance videos can be found below:
Demonstration of the streamlining updates
Demonstration of the response item and guidance updates
CONTINUUM Re-Entry Interview Script Enhancement (RISE)
Version 3.0 also implements the RISE, for patients who are in a controlled environment and will soon proceed to re-entry back into the community. The RISE re-frames questions for the controlled environment context so that patients are prompted to respond using more appropriate timeframes:
- past (prior to entering the controlled environment)
- present (within the controlled environment)
- future (after community re-entry)
The RISE enhancements are only applicable for agencies that have specifically asked to enable the functionality. If your agency has patients who are in a controlled environment and will soon proceed to re-entry into the community, please reach out to CONTINUUMSupport@FEIsystems.com to discuss enabling the RISE. A guidance video demonstrating the RISE functionality will also be made available on the ASAM CONTINUUM website for applicable agencies.
Version 3.0 Updated Item Changes
- A.a.0090: This item now asks whether the patient was released from jail or prison within the past 6 months, but no longer asks whether the patient has been in a penal or chronic care setting within the past 14 days. The update to this item aligns it with current federal regulations for opioid treatment program admission.
- B.a.0100, B.a.0120: These items now ask whether and when the patient has been tested for sexually transmitted diseases and tuberculosis. A “Not tested/not sure” response option also has been added to the tuberculosis question (B.a.0120). If an interviewer selects “Not sure/possibly” for B.a.0100, or “Not tested/not sure” for B.a.0120, the Assessment Report will include language alerting the clinician that the patient may need testing. These responses will also be included in the “Problem List” of the Narrative Report.
- B.a.0180: This item has been updated to include SSI and disability payments in the query of possible payment sources for disabilities.
- B.a.0460: This item (“…reemergence of acute symptoms that can be safely addressed only in a medically-monitored setting?”) now has a new instruction stating that a response of “yes” may escalate the Final Level of Care intensity and/or require Biomedical Enhanced Services (BIO).
- B.a.0010: The question structure and instructions to the interviewer (“What physical or medical problems have you had…?”) have been revised so that the interviewer is now asking the question to the patient directly.
- D.a.0010: The question structure and instructions to the interviewer (“Which substances have you had problems with?…”) have been revised and expanded to focus more on problems related to use and priorities rather than listing all substances the patient has ever used. Also, in the drug category response options, ecstasy has been reclassified as a hallucinogen.
- D.v.0130: The first response option for this item has been edited to read “Has detailed & comprehensive sense of the role of treatment vs. need for personal efforts – OR no relapse risk likely.” This additional help text has been added to the information icon for this item to help guide the clinician in choosing the appropriate response for a broader range of patient circumstances.
- F.a.0360: For this item (“How much help will this person (or these persons) need to assist…?”) the blue “i”nformation button now provides a detailed explanation for each response option.
- F.a.0040: This item, which asks whom the patient lives with, now allows users to endorse multiple response options.
- F.a.0460: This question asks whether the patient is able to locate and get to community resources safely. The response options now include more nuance: “No or unknown” and “Yes (or patient is remaining in a residential or controlled environment where resources are available)”.
- G.a.0030: The question has been re-worded so that the clinician asks up front about difficulties with mood, sleep, energy, nerves, eating, thinking, memory, or getting along with others. This change will allow the clinician to begin collecting data about psychological and emotional symptoms. Additionally, “Cognitive delays (developmental delays or borderline mental function)” has been added as a response option for this item.
- a.1660, G.a.1670, G.a.1580, G.a.1590, G.a.1600, G.a.1620, G.a.1630, G.a.1640G.a.1660, G.a.1670: More detail has been added to the response options for items about suicidal attempts (gestures), and help messages have been added to these items, to help guide the clinician in selecting the appropriate response.
- G.b.0180, G.b.0270: Response options for these items have been updated to include “No or not applicable.”
- G.b.0240, G.b.0270: Blue “I”nformation button help messages have been added to these items.
Version 3.0 Streamlining Updated Item Changes:
- a.0060: This item now has an additional response option of “Prison.”
- a.0010: In order to substantially shorten and streamline the interview, if the patient does not have any medical conditions, most of the medical section will now be suppressed.
However, several key medical questions related to pregnancy, infectious disease and/or medical conditions that could present risk during withdrawal management will remain in the assessment. - a.0430, B.a.0450, B.a.0460: These medical questions are gaining an “Unable to assess” response option. If the user selects this option for any of these items, the items will appear in the “Problem List” section of the Narrative Report; and an alert will appear in the Assessment Report stating that the interviewer was unable to assess what the question is asking, and recommending further medical evaluation.
- f.0010, D.f.0020, D.f.0030, D.f.0040, D.f.0050, D.f.0060, D.f.0070, D.f.0080, D.f.0090, D.f.0100, D.f.0110: In order to substantially shorten and streamline the interview, these CINA scale questions will be suppressed if the patient reports no use of opioids in the past 15 days (exception: up to 30 days for methadone or buprenorphine, since these are longer lasting agents).
- h.0050: This question (“…readmission: Does the program physician judge Opioid Treatment Services (OTS) readmission to be medically indicated?”) will be suppressed if the patient has not previously completed at least 6 months of Opioid Treatment Services with voluntary withdrawal management.
- k.0010, D.k.0020, D.k.0030, D.k.0040, D.k.0050, D.k.0060: These CIWA scale questions will be suppressed if the patient reports no use of alcohol or sedatives in the past 15 days.
- r.0040: This item about nicotine route of administration now includes “Smoke/Vape.”
- w.0100: This question, which asks whether the patient is displaying signs of withdrawal, will be suppressed if the patient reports no use of any drug category within the previous 15 days (30 days for methadone/buprenorphine).
- a.0020, F.a.0070, F.a.0100, F.a.0110, F.a.0130, F.a.0140, F.a.0360: To substantially shorten and streamline the interview, these questions, in the “Legal” and “Family and Social History” sections, will now be suppressed for patients who report no history of alcohol or other drug use.
- a.0070, F.a.0110, F.a.0130, F.a.0140: These questions will now include an additional response option of “Unknown.”
- a.0550: This question, which asks how much staff support is needed for a patient transitioning back to the community, will now include a new response option “Needs contact about once per month” for greater specificity.
- a.0030: To substantially shorten and streamline the interview, if the patient endorses no psychological or emotional problems in this item, many of the psychological symptom questions that follow will now be suppressed.
- a.0090, G.a.0130, G.a.0170, G.a.0210, G.a.0250, G.a.0290, G.a.0330, G.a.0370, G.a.0410, G.a.0450, G.a.0490, G.a.0530, G.a.0570, G.a.0610, G.a.0650, G.a.0690, G.a.0730, G.a.0770, G.a.0810, G.a.0850, G.a.0890, G.a.0930, G.a.0970, G.a.1010, G.a.1050, G.a.1090, G.a.1130, G.a.1170, G.a.1210, G.a.1250, G.a.1290, G.a.1330, G.a.1370, G.a.1410, G.a.1450, G.a.1490, G.a.1530, G.a.1570, G.a.1610, G.a.1690: To substantially shorten and streamline the interview, for patients who endorse past-24-hour psychological or emotional symptoms, but deny any alcohol or other drug use, questions asking whether these symptoms were due to alcohol or other drug use or withdrawal will now be suppressed.
- b.0150: To substantially shorten and streamline the interview, this item (whether risk of harm to self or others is related to alcohol or other drug use) will now be suppressed for patients who endorse psychological/psychiatric risk but deny any use of alcohol or other drugs.
- a.0030: To substantially shorten and streamline the interview, this question (how soon the patient is likely to respond to withdrawal management care) will now be suppressed for patients who deny any use of alcohol or other drugs, or who deny use within the past 15 days (30 days for methadone).
- a.0090, H.a.0100, H.a.0110, H.a.0120, H.a.0130: To substantially shorten and streamline the interview, these medical questions, which appear in the Interview Completion section, will now be suppressed if the patient previously denied having any medical problems.
- a.0190: This question (whether the patient would recommend the treatment program to a friend in need of help), will have an additional response option of “Not applicable” so that it applies to patients who have not yet begun treatment.
User Interface Updates:
- Item Numbering Convention for ASAM CONTINUUM & Triage: A new number convention was added to allow interviewers to more easily identify specific questions (e.g., for supervision purposes, or for bug reporting) and for researchers to more easily identify items within the decision logic.
- Assessment: Multiple Formatting Issues: Drug and Alcohol Section: Headers and dropdown responses in the Drug and Alcohol section now wrap, so they don’t appear to go offscreen or require horizonal scrolling when the user zooms in.
- Save Button Visible on Every Screen: Save button is now always visible on every screen, rather than only appearing after a change has been made in the assessment.
- Add Message Banner to ASAM CONTINUUM and CO-Triage Screens: A banner has been added to the top of the ASAM CONTINUUM and CO-Triage screens to communicate important messages to end users.
- ASAM CONTINUUM: Make Auto-save Function for Assessments Transparent: ASAM CONTINUUM now automatically saves the assessment every 3 minutes or after each time the user navigates to a new section within the assessment. The last save time is now displayed at the bottom of the screen.
- Incomplete Items More Clearly Identified: In addition to the completion percentage for each section in the side navigation, ASAM CONTINUUM & CO-Triage assessments will now identify incomplete required questions. The subsection header will be in red font, and the field itself will be highlighted red.
ASAM CONTINUUM Assessment Updates:
- Add Question to Legal Section Asking About Illegal Activity in the Last 30 Days: The following question was added in order to streamline data entry in the Legal section: “Have you ever been arrested or charged for any reason?” (Includes misdemeanors, driving under the influence, and other major driving violations, e.g., reckless driving, speeding, no license, etc.)
- Add Validation for Abstinence Question on Additional Addiction and Treatment Items Screen: Validation added in Additional Addiction and Treatment Items section to make sure that the response to the “when last used” question for a specified drug does not contradict the response to the abstinence question for the same drug.
- Add Response to Living Arrangement Question: In the Family and Social History section, the following response option was added: “Alone with Child/Children” to the dropdown for “What are your usual living arrangements (past 3 years)?”
- ASAM CONTINUUM: Capture User Who Submitted Assessment: Both ASAM CONTINUUM and CO-Triage now capture the user who submitted the assessment to determine if a different user initiated the assessment than the user who submitted the assessment. The information is not visible on screen but will be available for EHRs to pull back for reporting purposes.
- Narrative Report: Spelling Error Fixed in ASAM Dimension 3 on Problem List: On the Narrative Report problem list, the word “community” is now spelled correctly for the psychological section interviewer rating question “Is the patient able to safely access the community for work, education, and other community resources?”
- Item Text Changes: Updated question text to provide additional clarification to the interviewer to increase more accurate responses.
- All CUAD Questions Now Required in CONTINUUM: All questions related to the Chemical Use, Abuse, and Dependence (CUAD) scale are now required in ASAM CONTINUUM, for more accurate DSM 5 diagnosis information.
Report Updates:
- ASAM CONTINUUM Summary Report Dimensional Analysis Now Lists All the LOCs That Meet the Criteria: The summary text and ASAM dimensional analysis grid on the ASAM CONTINUUM Summary Report now list all Levels of Care and qualifiers that meet the criteria.
- Add Response from Text Box (ASIm1a and 6a) to the Narrative Report: In the Medical History section, question label was updated from “Describe the medical problems indicated in the above categories” to “Please describe specifically.” Response to the question “Is the patient currently showing symptoms or signs of intoxication” was also added to the Narrative Report.
- ASAM CONTINUUM and CO-Triage Reports: Time and Date Stamp: ASAM CONTINUUM and CO-Triage reports previously reflected the date and time in Eastern Time. The reports now reflect the user’s time zone.
Co-Triage Assessment Updates:
- Incorrect Spelling of Possibly Located in Co-Triage Assessment: On the Co-Triage Dimension 6 – Recovery/Living Environment section, the word “possibly” is now spelled correctly in the response dropdown to the question “Do you have any problems walking or getting around that would make it difficult to attend treatment?”
Level of Care Recommendation Updates
Version 2.8 updates bring the ASAM CONTINUUM algorithm closer in line with the latest version of the ASAM Criteria textbook and improve the accuracy of recommendations for several levels of care, including:
- Increases in OTS, Opioid Treatment Services by distinguishing between the following cases: patients who meet federal methadone regulations; patients who do not meet regulations but are candidates for other opioid treatment services; patients who need both OTS and another level of care; and patients who wish to continue or resume OTS
- Increases in Level 3.1 outputs
- Decreases in Level 4, Medically Managed Intensive Inpatient Services
- Improvement in Level of Care specificity related to patient supervision and structure, cognitive function, OTS need, and acute psychiatric risk
Medical History Section Updates
- Blood pressure and heart rate questions are no longer required to accommodate interviewers that are unable to capture blood pressure and/or heart rate measurements during administration of the assessment
ASAM CONTINUUM Assessment Updates
- Added popup help for Question in Completion Section: In the Completion section, the question “Does the patient exhibit any symptoms that would be considered life-threatening AND are related to alcohol or drug use?” now provides a helpful pop-up to guide an evaluator to answer more accurately. The pop-up states: “Immediate and life-threatening symptoms will result in a direct transfer to a medical hospital. Please confirm your response with a qualified medical provider.”
- Added Fentanyl to the Used Substances Screen: Interviewers asked that in the Drug and Alcohol Section of the assessment, Fentanyl should be added as an option to the Used Substances screen.
- Added the ability to select multiple options to the first question in the Review section: In response to end user requests, in the Review Section of both CO-Triage and CONTINUUM, users now have the ability to select a primary category of final disposition, and then multiple additional categories of final disposition. This will allow users, for example, to select Level OTS-Opioid Treatment Service in combination with any other Level of Care.
- Unanswered questions should now remain blank instead of defaulting to “No”: Previously, after an assessment was submitted, all unanswered questions were defaulted to “No” or “False” when the assessment was reviewed or printed. With this update and going forward, unanswered questions will remain unanswered upon review or printing.
- Question sequence improved in the Family/ Social History Section: In the Family/ Social History section, after review, it was found that the questions should appear on the screen in a different order to best evaluate the patient. This has been corrected so there is a change to the sequence in which the questions appear.
- Question Response items are differing in CONTINUUM vs Co-Triage: The responses in the review section have been synchronized between CONTINUUM and CO-Triage.
Report Updates
- Narrative Report: Question with multiple answers needs has been revised for Narrative Report: The question “Has the patient responded to appropriate recent efforts by the prescriber to maintain does at therapeutic levels?” previously required the answer to be positive for all 7 relevant substances in order for this to appear on the problem list. This issue has been addressed so that if the answer is positive for one or more substances, the item will still be added to the problem list.
- Narrative Report: Output Error in Recovery/Living Environment Section: In response to end user comments, the problem list was updated so that negative responses are not inappropriately reflected as potential problems.
- Narrative Report: Answers with 0 or No are answered as “He/She does not remember or will not discuss…”: In response to end user requests, in the narrative report, questions left unanswered or answered with 0 will no longer be represented by the phrase: “”He/She does not remember or will not discuss…” Each related phrase has been customized to be more accurate for an unanswered or 0 response.
User Interface Updates
- EULA screen is displaying “This site can’t be reached” when “I Agree” is clicked: New users will no longer receive an error message upon accepting the terms of the End User License Agreement.
- The system scrolls the user back to the top of the screen upon clicking Save: Previously, users were redirected to the top of the current section of the assessment when they clicked the Save button. This update allows users to save and retain the same position in the assessment section.
- Navigation requires multiple clicks to access a subsection: At the request of end users, the user interface has been updated to make navigation through sections and sub-sections easier, for a smoother process and a reduction of time needed to complete the assessments.
- Comment boxes are now resized in Print document: Previously, when users printed the CONTINUUM or CO-Triage assessment, comments boxes were cut off or sized wrong so that the comment was not fully visible. At the suggestion of end users, with this update, the comment boxes have been expanded to show all content when printing.
Important Clinical Updates:
- Gender options added into the client profile for CONTINUUM: This update implements CDC, National Academy of Medicine, and similar recommendations for high-quality, patient-centric care, by:
- Expanding CONTINUUM’s gender identity options
- Integrating items to capture sex-at-birth and patient-preferred gender pronouns
- Allowing for pregnancy status to be collected on all individuals
- CONTINUUM – General Information & Legal Section – Sync jail and incarcerated question: This update improves data integrity in the General Information and Legal Information sections between two items that query the number of days the patient has spent in a controlled environment in the last month. If responses to these items do not match, the Interviewer will now automatically receive a notification. Additionally, the response value for number of days in the past 30 days will be restricted to between 0 and 30.
- Changes to Accommodate Abuse-Neglect of a Minor or Elder: This revision in the Family and Social History section improves the abuse and neglect questions by:
- Updating the wording of some items
- Adding blue “i” button help information associated with the change
- Improving the sequencing of these items
- Introducing a free-text field so the Interviewer can describe any risks
- Providing guidance and resources on duty to report requirements
- Updates to account for patient self-reported data: In response to the COVID-19 pandemic and needs for telehealth interviewing or interviewing by non-medical/nursing personnel, CONTINUUM now provides improved guidance on assessments collected via phone or videolink, including:
- Information icons for the CIWA and CINA withdrawal assessment items now include instructions to allow patient self-report in the event that the interviewer is unable to assess.
- The Narrative and Summary Reports specify that assessments collected via telehealth interview or by non-medical/nursing personnel will contain more patient self-reported data and that self-reported data may impact conclusions or recommendations
ASAM CONTINUUM Assessment Updates:
- Corrected typo found in Review Section: In the Review section, screen question I.a.0030 misspelled the word “received.” This has now been corrected.
ASAM CONTINUUM Assessment Updates:
- Corrected item numbering: Two questions in the Drug and Alcohol Other Substance Use section have been updated to correct the item numbering. Occupational activities has been updated to D.s.0210 and Family/Friends has been updated to D.s.0220.
Report Updates:
- A patient ID field has been added to all reports: To provide more clarity, all generated reports now display a Patient ID field in the header.
- Updated narrative report messaging for non-resolving cases: In order to assist with non-resolving cases, the language used to describe non-resolving cases has been updated to better reflect the reason the system could not determine an accurate level of care.
- Update #1: This change improves alignment between the ASAM CONTINUUM algorithm and the 2013 ASAM Criteria textbook: CONTINUUM no longer considers a risk of harm from others in the formula for current risk as that can imply a need for hospitalization or extended residential placement. This change reduces the frequency of recommendations of Level 4 and Level 3.3.
- Update #2: This change revises a measure of current risk of harm to self or others to improve level of care placement for patients with past psychiatric issues that are unlikely to recur. It reduces the frequency of recommendations for Levels 4, 3.5, and 3.3.
- Update #3: This change improves the rate of DSM-5 SUD diagnoses by adding a DSM-5 diagnostic question pertaining to continued use despite physical/psychological problems.
- Update #4: This change edits Dimension 1 decision rules to better specify psychiatric complications of stimulant use and improve the gradation sensitivity of symptom severity across levels of care. The result is less overuse of Levels 4 and 3.7.
- Update #5: This change resolves an internal conflict in the Dimension 3 decision rules for Level 1 COE.
- Update #6: This version alignment step simply updates the ASAM CONTINUUM algorithm’s Level 3.1 COC decision rules sequence from the ASAM PPC-2r Edition (2001) to match the subrule sequencing in the ASAM Criteria 3rd Edition (2013).
- Update #7: This change adds nuance to Dimension 3 decision rules within Level 3.1 to avoid excluding patients with some psychological severity that manifests only with active substance use and/or does not distract from the recovery process. The result allows more patients to receive recommendations for Level 3.1 instead of Level 3.1coc.
- Version 2.10.1 is a branch release for the current 2.10 version in production. ASAM CONTINUUM has been updated to accommodate interviews that occur via telephone or video Telehealth, and under circumstances where it is difficult to appropriately assess a patient’s medical status: i.e. withdrawal symptoms or medical care requirements. Several sections of the tool have been adjusted to accommodate this update, including General Information, Medical History, and the Drug and Alcohol sections. The Narrative and Summary Report have also been updated to reflect the data input in the assessment with these updates. With these updates, ASAM has released a guidance video demonstrating the changes you will see in the software. You can find the video on the ASAM CONTINUUM website through the link included here. The video link can also be accessed from the CONTINUUM home screen. On the bottom left hand corner of the CONTINUUM tool there is a button for “Update Notes.”
User Interface Updates:
- Click the new little blue circle “i” info buttons: Interviewers asked for helpful hints – so these are now added to many ASAM CONTINUUM assessment questions. They provide further details and instructions to assist the user while administering the assessment. Interviewers should click these for help – for less guessing & more knowing – about interpretation of a question, how to solicit a more targeted response, and how answers might affect placement.
- What’s been updated – now and in the past? Look on the left, blue Navigation Panel, and click the “Releases” tab to see Current and Previous Release Updates. Important: Users must review these Updates to take advantage of new features designed to streamline interviews and improve accuracy.
- Print Errors Now Fixed in Reports: When printing from an ASAM CONTINUUM Assessment, some answers were not appearing on the reports. These are now present next to the question asked. Some answers previously ran off the page – these now “wrap” so the entire answer appears in the document. Some questions that did not come up during the assessment were appearing on the printed version. These questions will no longer appear on reports.
- Microsoft Edge “Save” Function – Now Fixed: Previously, the assessment screen showed the “Last Saved” time as “invalid date”. This now shows “Last Saved” followed by the time and date the assessment was last saved.
ASAM CONTINUUM Assessment Updates:
- Transdermal Patch – New Option for Route of Administration: In the Drug & Alcohol Section, Heroin and Fentanyl Sub-section, users can now select “Transdermal Patch” as an option in the dropbox for the question, “How did you most often or most recently use heroin or fentanyl? (Indicate most serious route of use)”
Report Updates:
- Narrative Biopsychosocial Report – Now with Page Numbers: Page numbers and total number of pages are now shown on the Narrative Report at the bottom right corner of each page.
- Narrative Biopsychosocial Report – Better Wording: We’ve improved the grammar in the Employment section, Education, Training and Resources sub-section within the Employment section. If the patient answers “yes” to the questions, “Do you have a valid driver’s license” and “Do you have an automobile available for your use?” the statement will now read, “He/she has a valid driver’s license and access to a car which may help him/her in maintaining employment.”
- Narrative Biopsychosocial Report – Patient Relationship Details Now Corrected: In Family/Social Section, Recovery Environment and Social Contacts sub-section, we have improved the text output for the question “Who is the person (or persons) with whom you have had contact during the past 4 months and who has been most important to you?” For example, if the patient responded with “sexual partner/spouse,” the report will now state, “{Patient name} reports that in the past 4 months she/he has had close relationships with her sexual partner/spouse.”
- Summary Report Dimensional Analysis – Corrects Level 4, Biomed: The Levels of Care chart no longer includes “Level 4, Biomed”, since Biomed is a service qualifier only for Level 3.
Expand all
Collapse all
